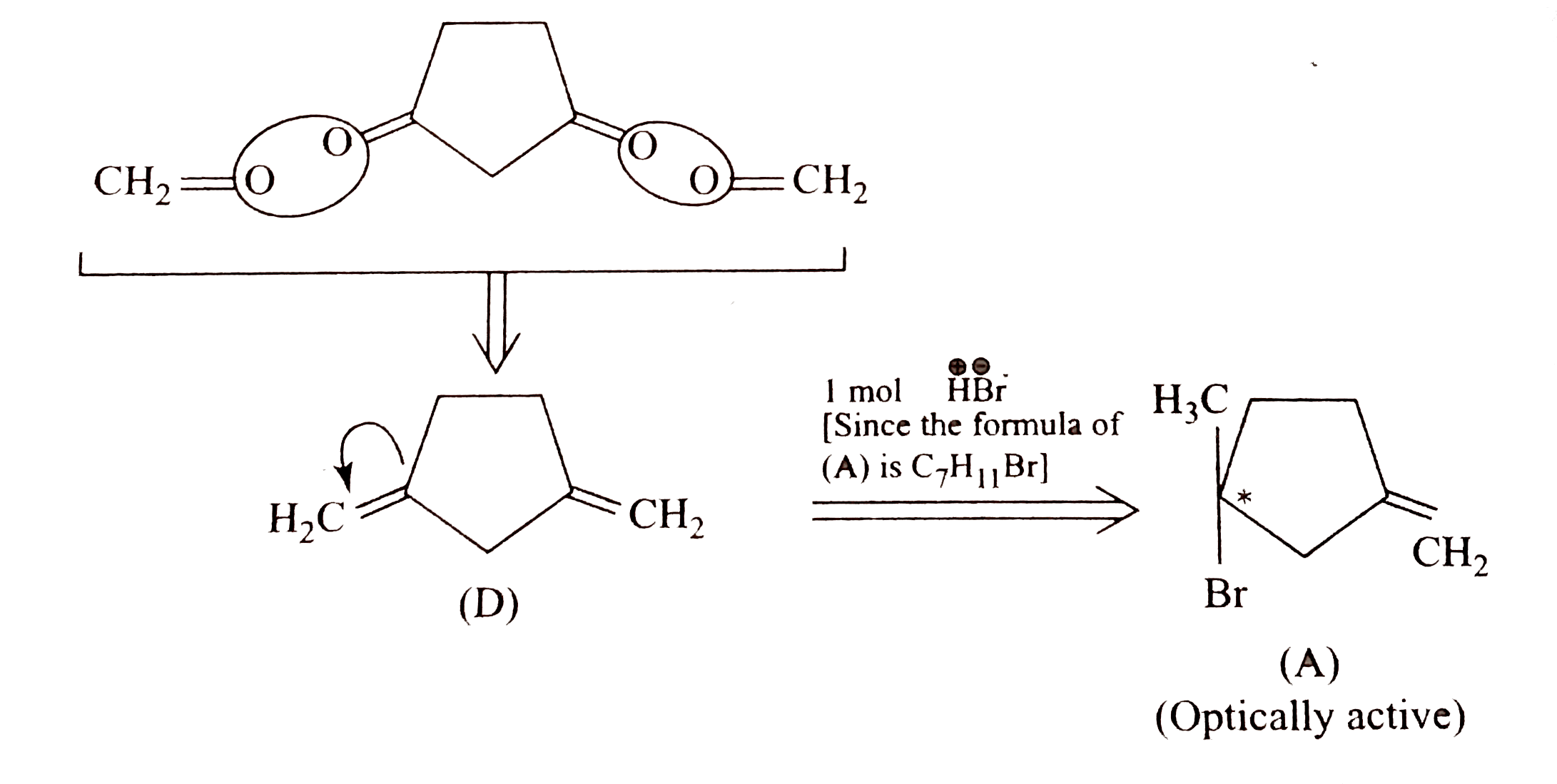
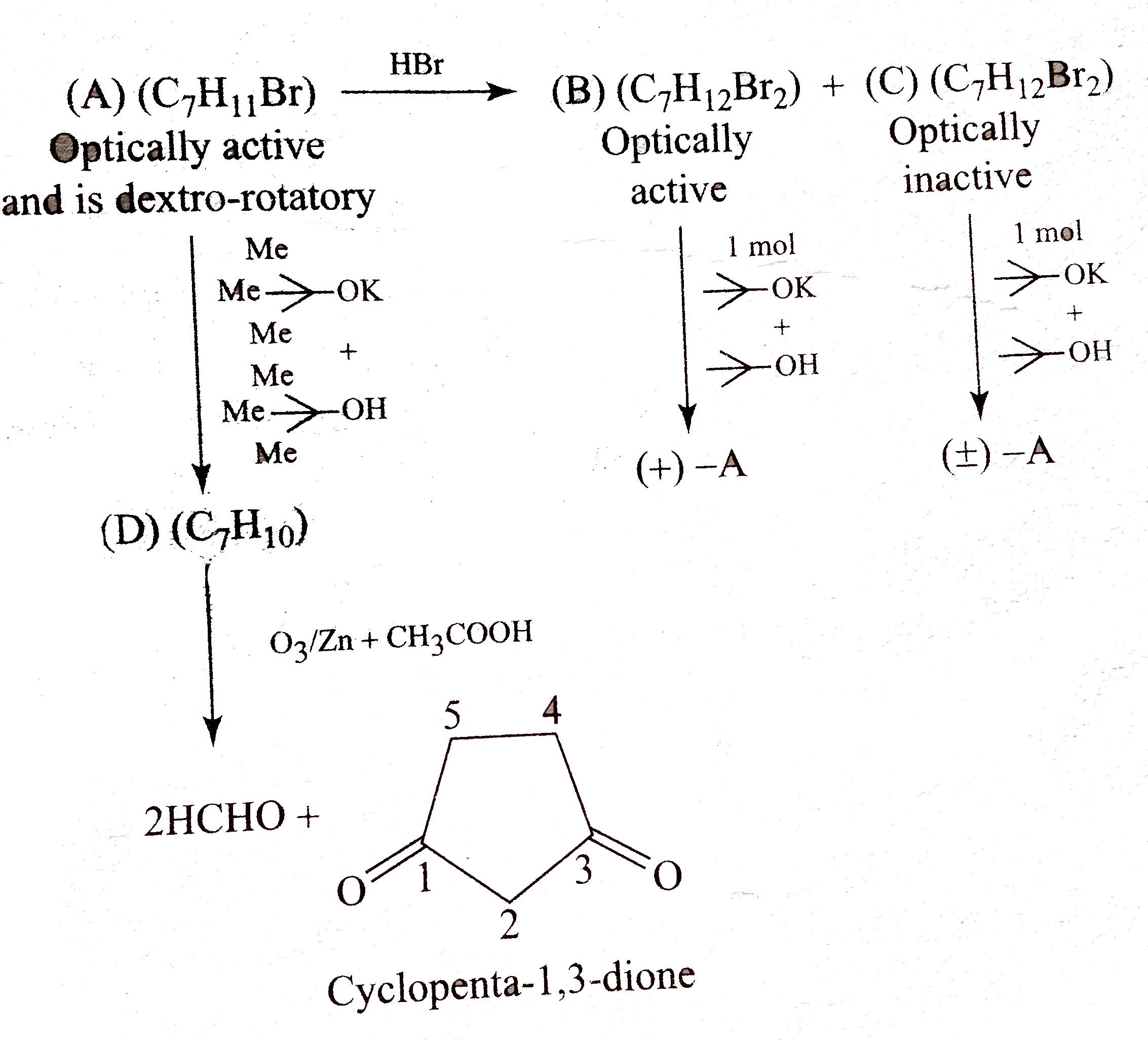Text Solution
Verified by Experts
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise linked Comprehension Type|38 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct Answer Type|41 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise SOLVED Example|62 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKENES AND ALKADIENES-EXERCISES
- Identify (A) to (I)
Text Solution
|
- Identify (A) to (F) .
Text Solution
|
- Give the major product and mechanism involved in the following reactio...
Text Solution
|
- Identify (A) to (C).
Text Solution
|
- Identify (C).
Text Solution
|
- Explain the following reaction . Why (A) mainy give (B)(1^(@) al...
Text Solution
|
- Propose structures for the hydrocarbon that gives the following produc...
Text Solution
|
- Write the structural formulae for the compounds that yield the followi...
Text Solution
|
- Give the products formed on reduction with LAH and oxidation with H(2)...
Text Solution
|
- Give the products formed by the oxidation of hot KMnO(4). a. Cyclobu...
Text Solution
|
- Deduct the structural formul of a compound (A) (C(10)H(16)) the gives ...
Text Solution
|
- Write the resonance structures of the given compound.
Text Solution
|
- Explain the following observation : a. When pent -1 en -4- yneistre...
Text Solution
|
- Give the stereochemical forculae for (A),(B)(C), and (D) and also give...
Text Solution
|
- Identify (A) to (E)
Text Solution
|
- Identify (A) to (E).
Text Solution
|
- A mixture of ethane (C(2)H(6)) and ethene (C(2)H(4)) occupies 40 L at...
Text Solution
|
- Draw the structure of aniline
Text Solution
|
- Give the stereochemical structures of B and C. 2,overset("Erythro-"...
Text Solution
|
- Give the stereochemistry of the reation (B).
Text Solution
|