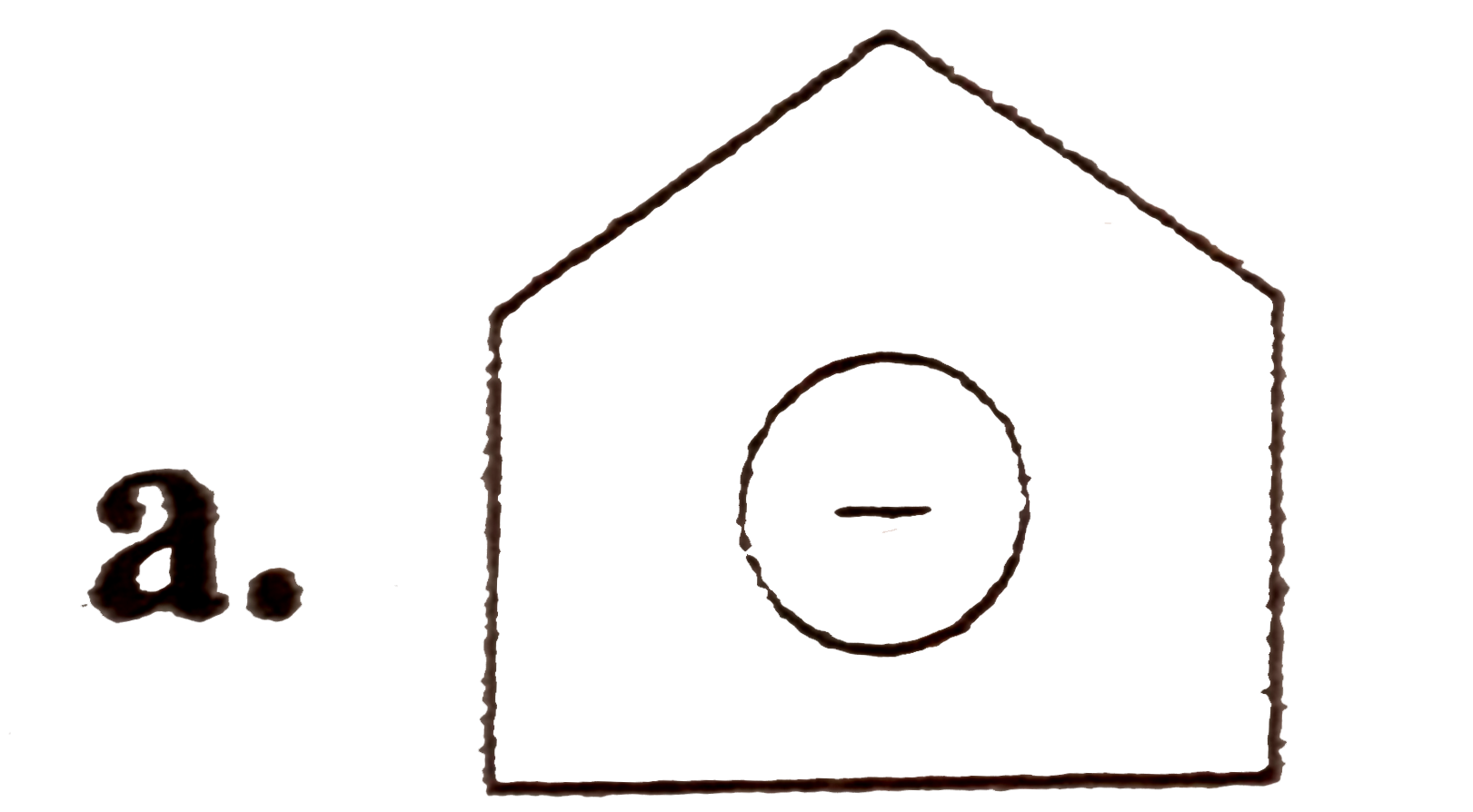
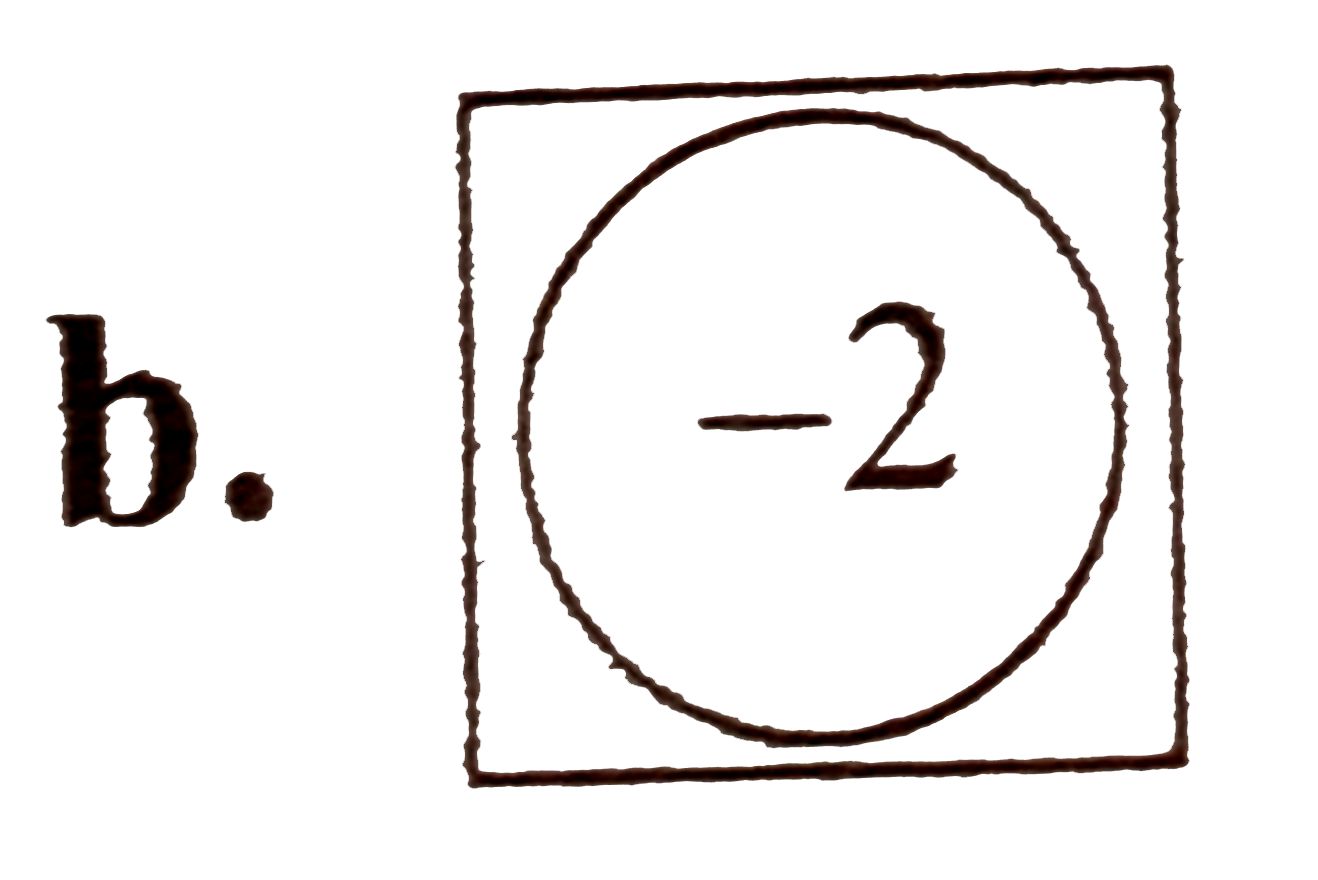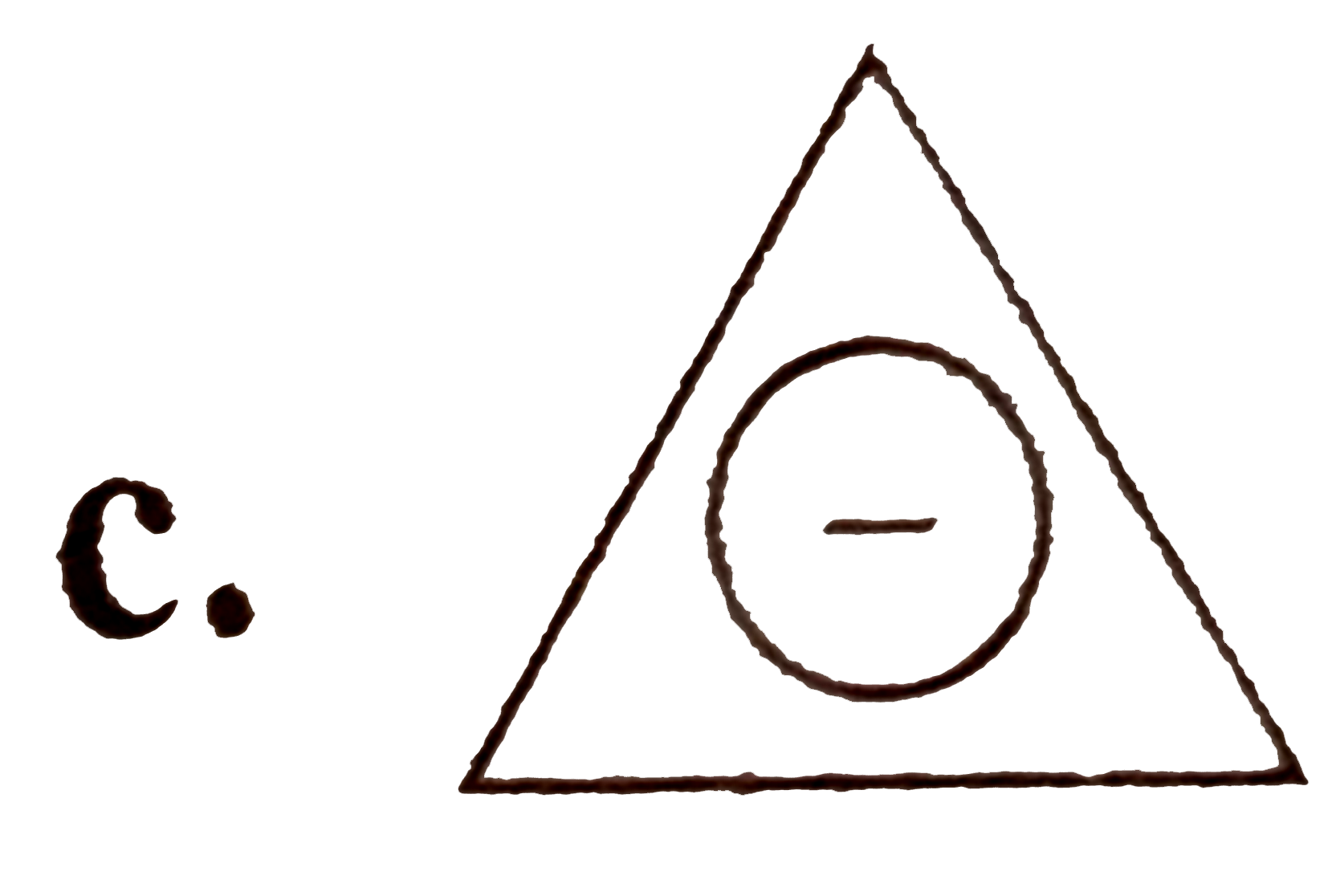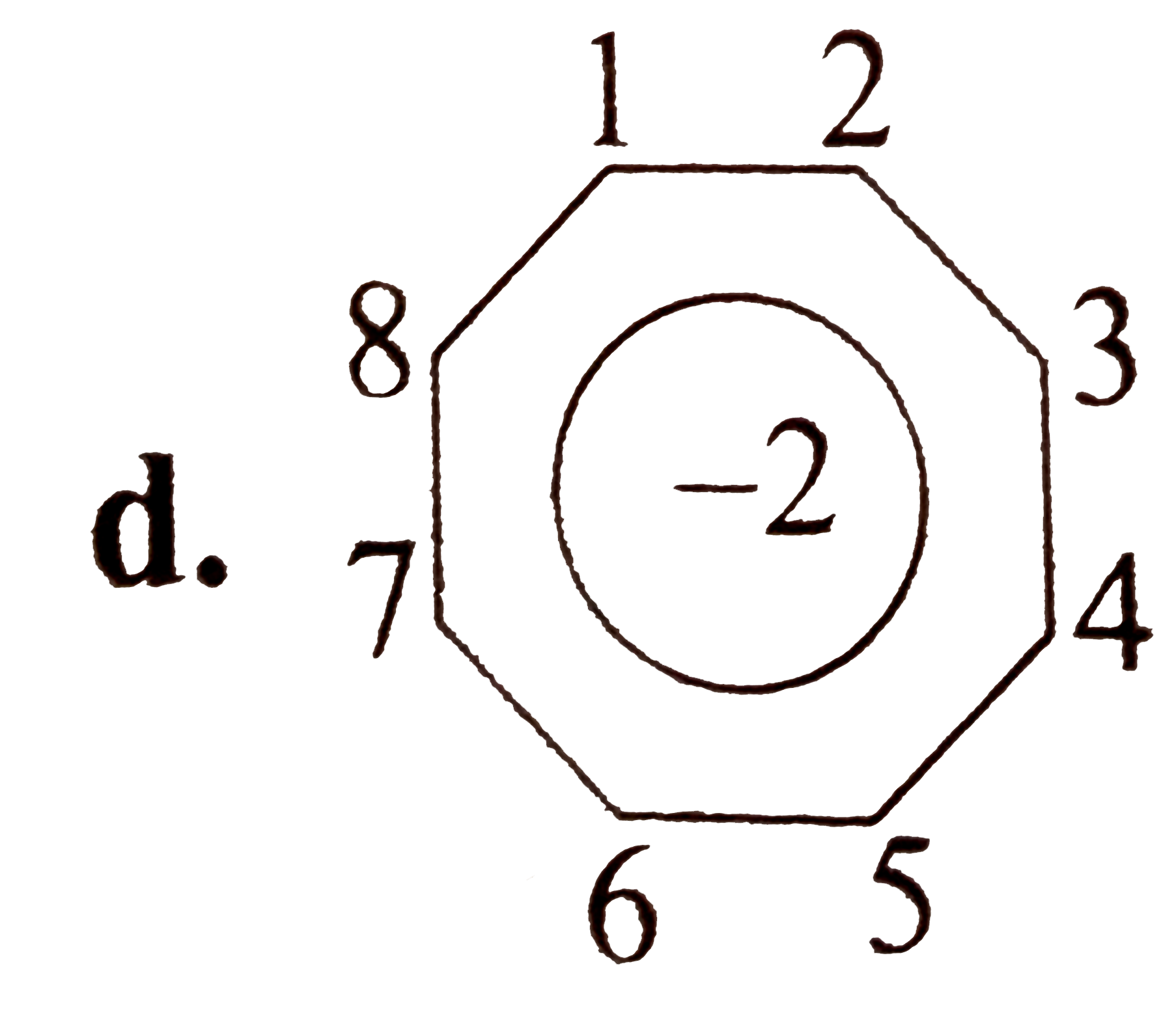A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives(ASSERTION-REASIOING)|4 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the Blanks|3 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct Answer Type|41 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKENES AND ALKADIENES-Single Correct Answer Type
- Which of the following is the most stable species ?
Text Solution
|
- Which of the following is non - aromatic in nature ?
Text Solution
|
- Which of the following species is least stable ?
Text Solution
|
- Which of the following is anti- aromatic in nature ?
Text Solution
|
- Intermediate (B) and Product (C) are :
Text Solution
|
- Which statement is correct about cyclopentadienyl anion ?
Text Solution
|
- In which of the following, the DeltaH(h)^(@) is maximum ?
Text Solution
|
- Compound (C) is :
Text Solution
|
- The decreasing order of reactivity towards electrophilic addition (e.g...
Text Solution
|
- (B)overset(2mol)underset(Cl(2)+hv)(rarr) (C)overset(2mol)underset(Alc....
Text Solution
|
- Which of the following reagents can be used for the reaction given bel...
Text Solution
|
- Compound (A)(C(5)H(10)) does not dissolve in cold conc. H(2)SO(4). W...
Text Solution
|
- Product (B) and name of the reaction in the formation of (B) are :
Text Solution
|
- Which statement is correct ?
Text Solution
|
- Compound (B) is :
Text Solution
|
- Which reagents could be used to carry out the following reaction ?
Text Solution
|
- Which reagent is used for the conversion of (A) to (B) ?
Text Solution
|
- Product (B) is :
Text Solution
|
- Which reagent cannot be used for the above conversion ?
Text Solution
|
- Which one is the most easily dehydrated ?
Text Solution
|