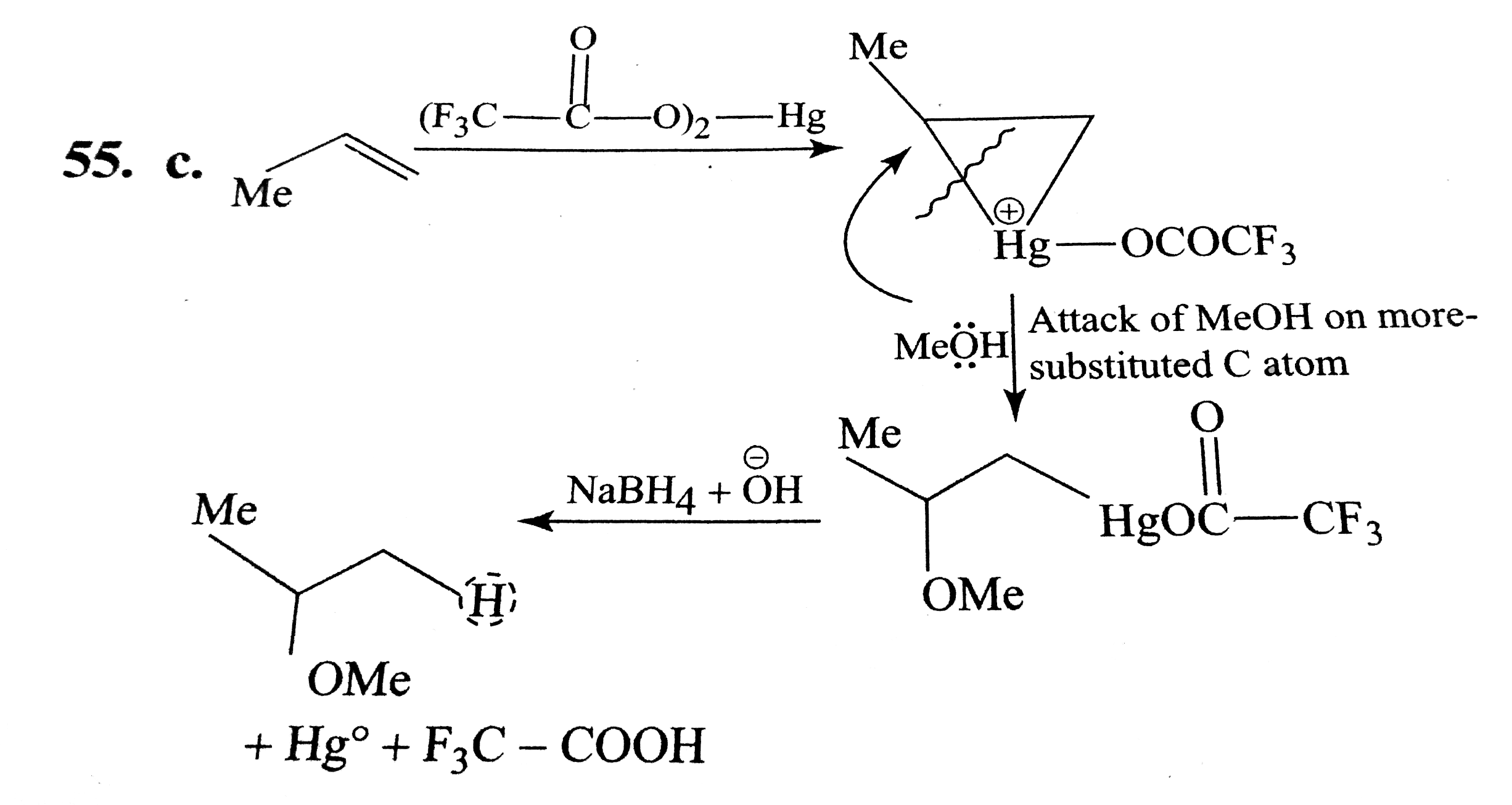
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives(ASSERTION-REASIOING)|4 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the Blanks|3 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct Answer Type|41 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKENES AND ALKADIENES-Single Correct Answer Type
- (A) and (B) are :
Text Solution
|
- (A),(B),and (C) are:
Text Solution
|
- (A)overset((i)BD(3)//THF)underset((ii)CH(3)COOH)larrPenten eoverset((i...
Text Solution
|
- Compound (A) are :
Text Solution
|
- Which is the wrong statement about oxymercuration - demercuration ?
Text Solution
|
- (A), (B), and (C) are :
Text Solution
|
- Which is the wrong statement ?
Text Solution
|
- Which statement(s) is/are correct ? i. In oxymercuration and demercu...
Text Solution
|
- Arrange the following alcohols in the decreasing order of dehydratio...
Text Solution
|
- (A) is :
Text Solution
|
- A is :
Text Solution
|
- Which statement is true ? i. The product is ii. The product is ...
Text Solution
|
- Identify the product
Text Solution
|
Text Solution
|
- The product is :
Text Solution
|
- Define halides
Text Solution
|
- The product is :
Text Solution
|
- The product (B) is :
Text Solution
|
- Give the decreasing order of reactivity of Diels - Alder reactions f...
Text Solution
|
- H(2)O(2)+overset(Θ)(O)H B and C are:
Text Solution
|
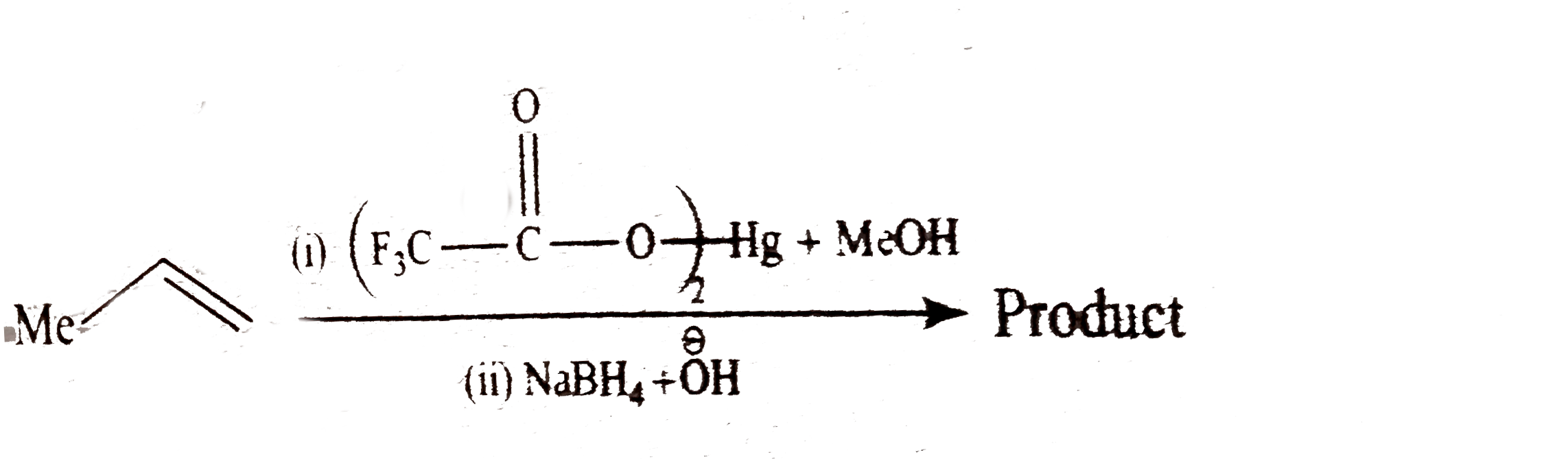
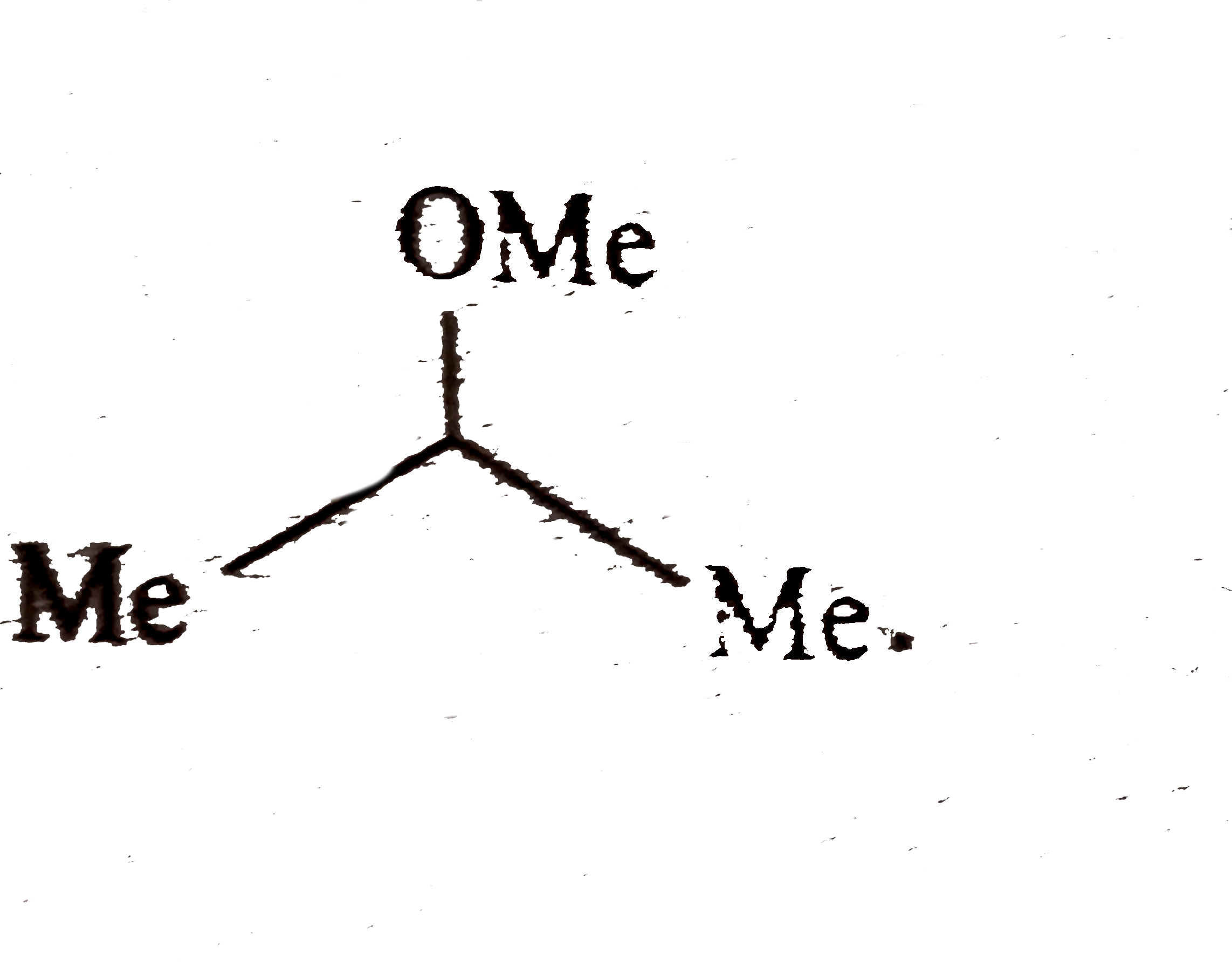
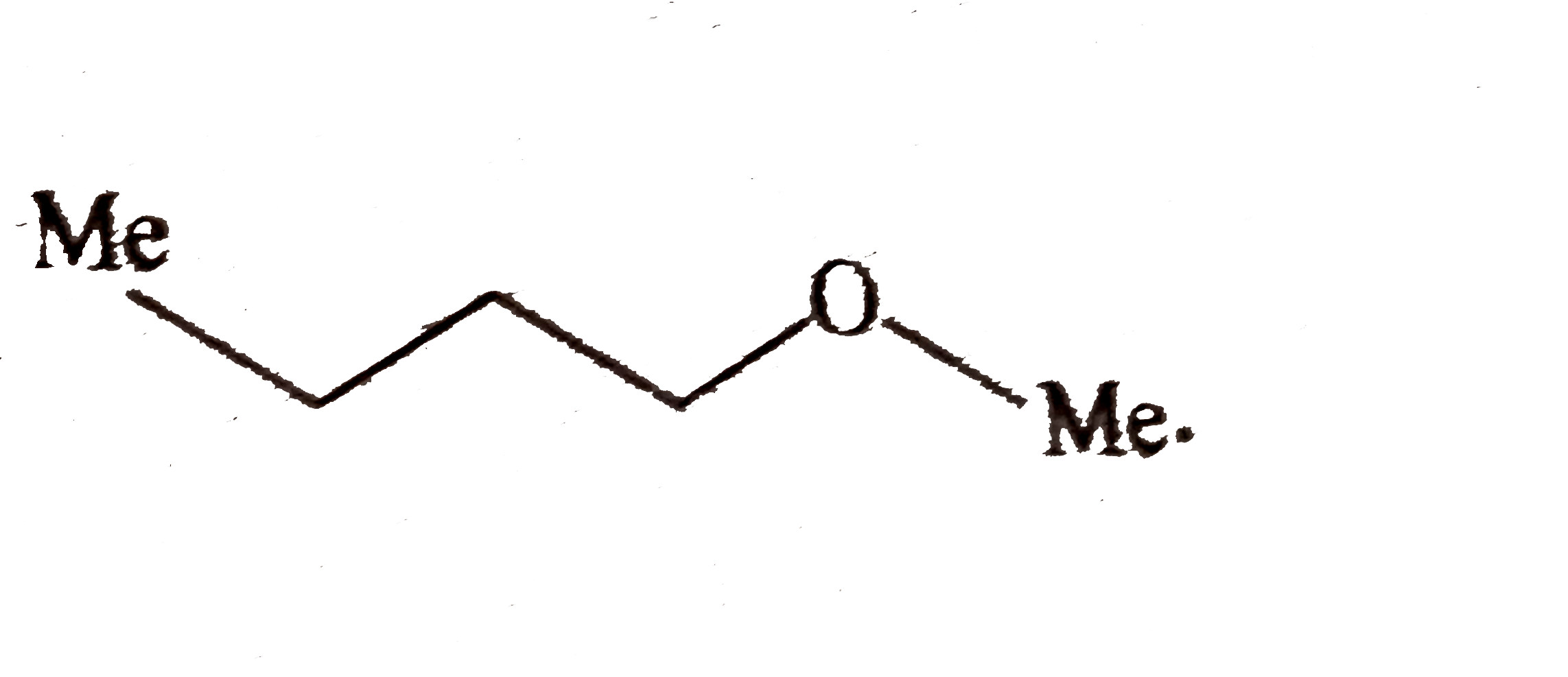
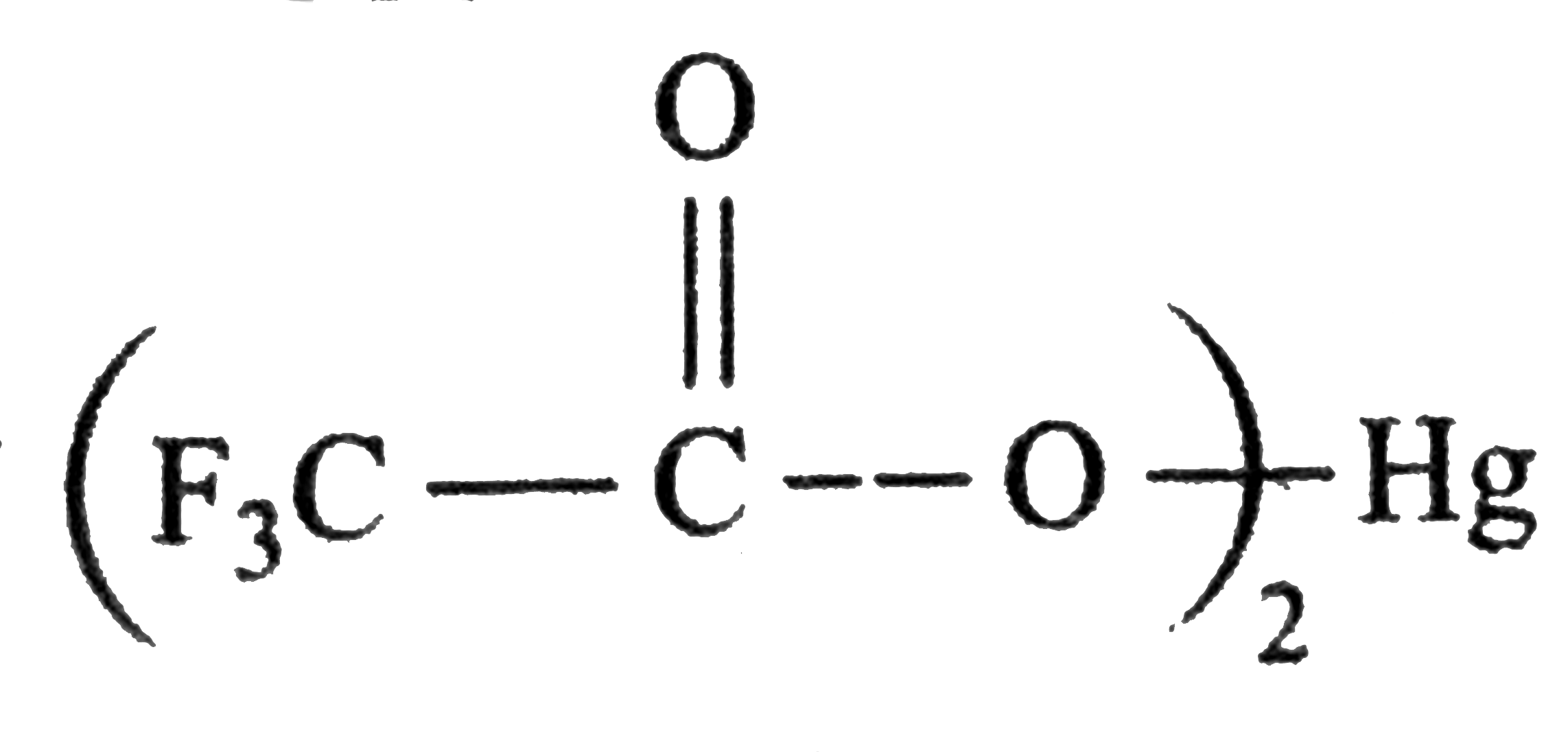 and `ROH(` a nucleophilc solvent `)` and not rearrangement.
and `ROH(` a nucleophilc solvent `)` and not rearrangement.