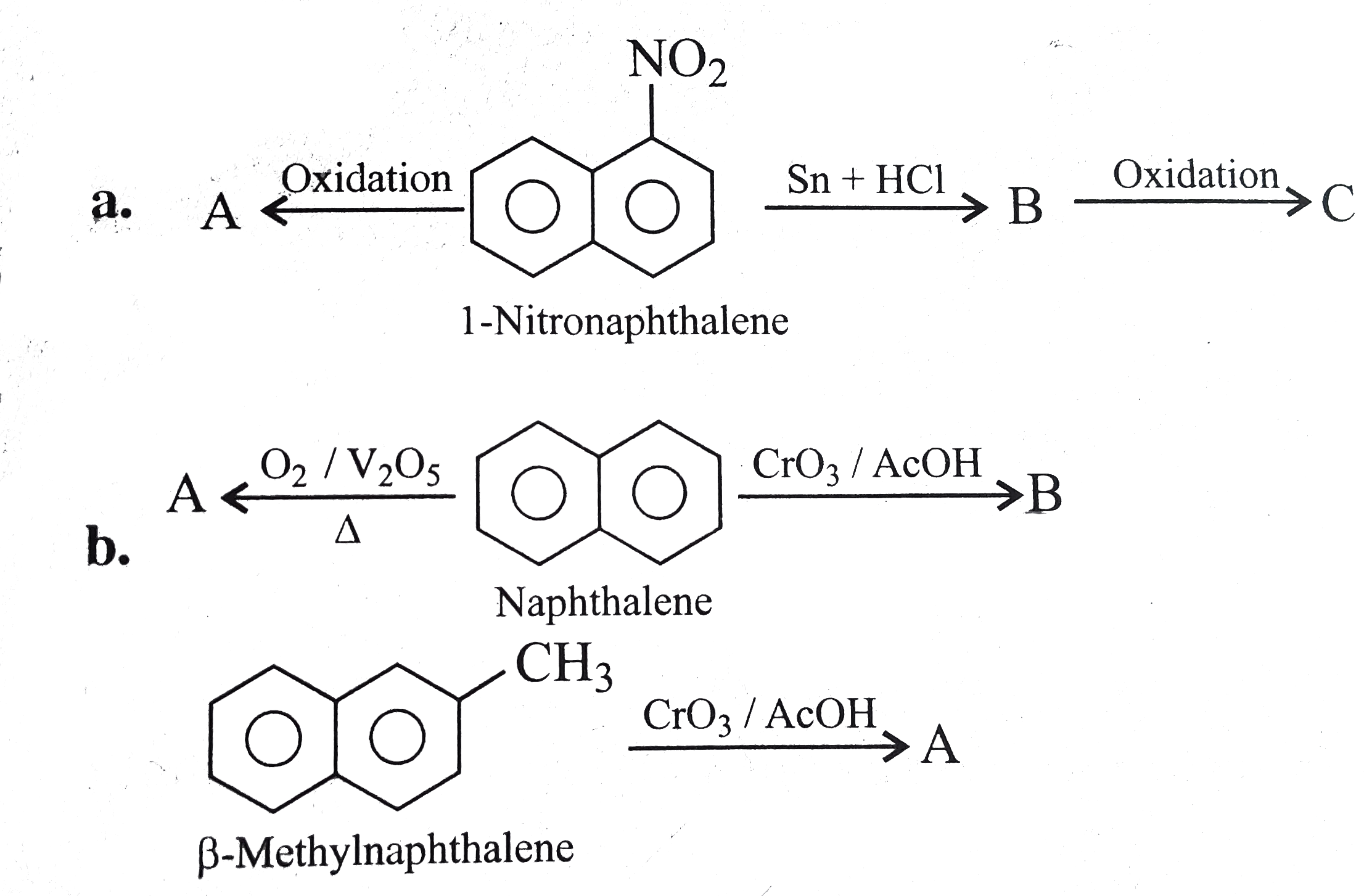
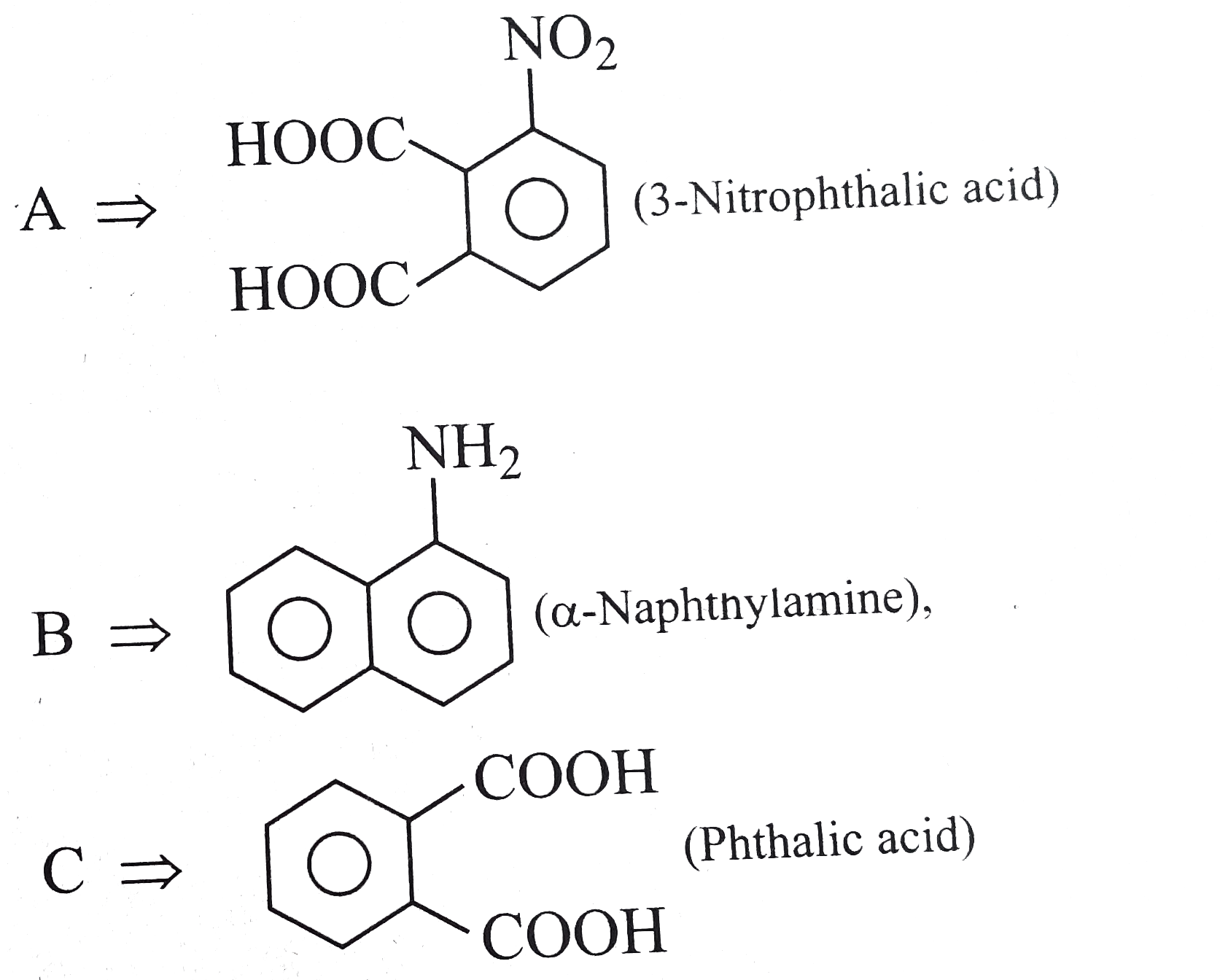
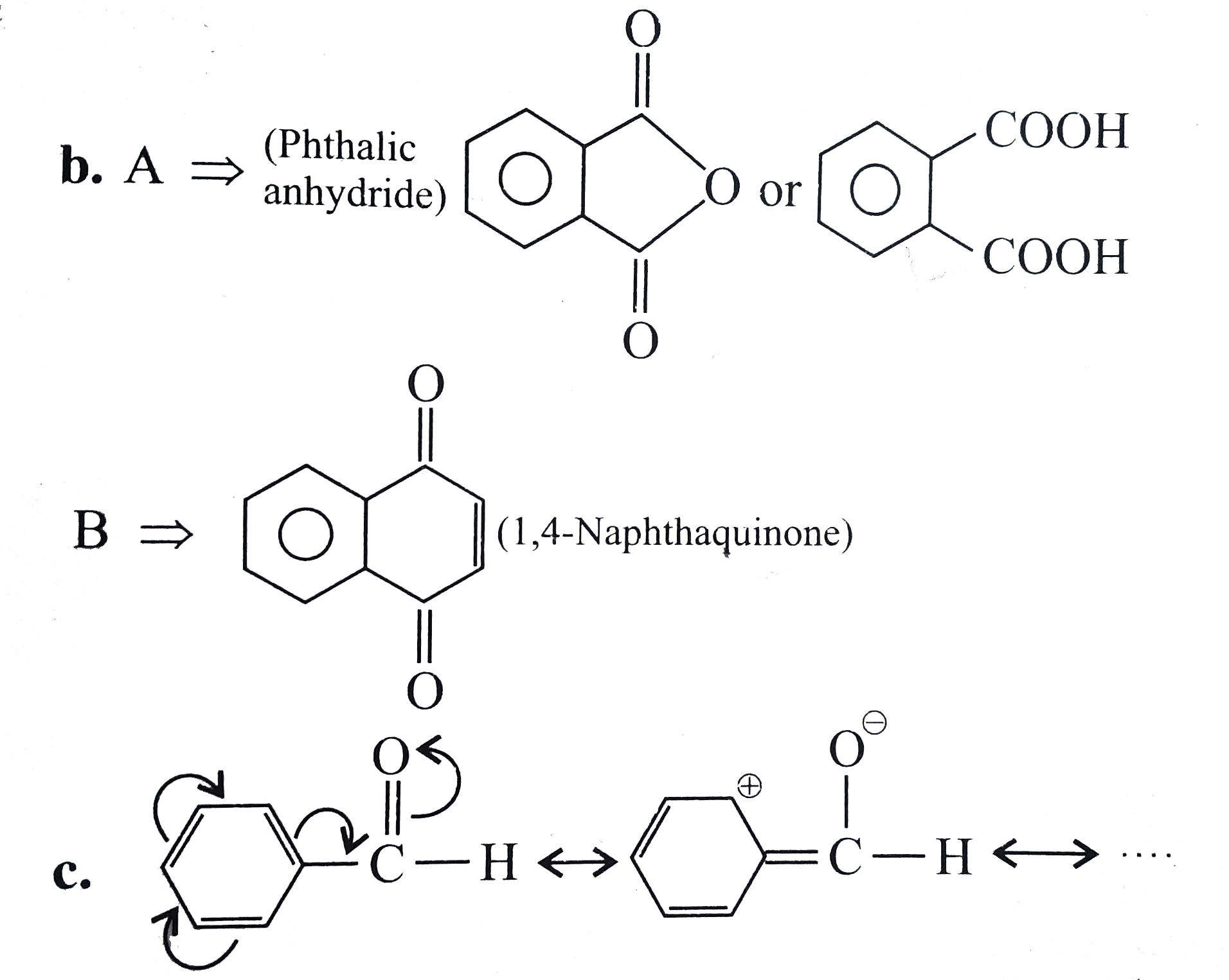
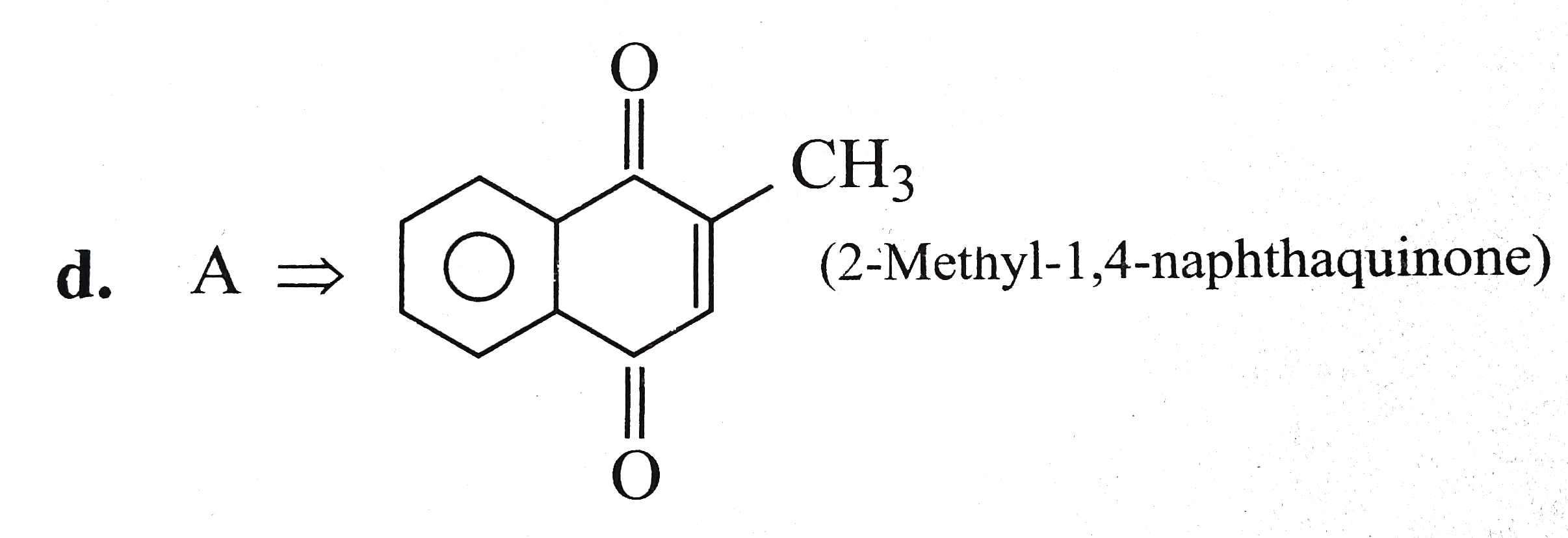
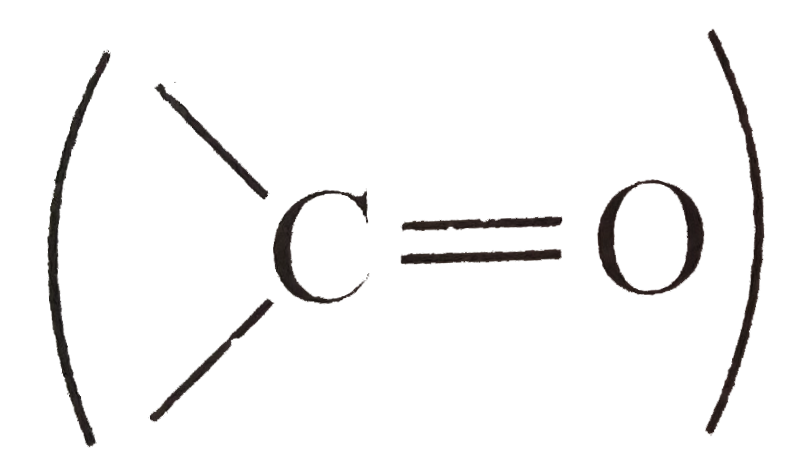Text Solution
Verified by Experts
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|6 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise EXAMPLE|1 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 Videos
CENGAGE CHEMISTRY ENGLISH-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-SUBJECTIVE TYPE
- Complete the following reaction
Text Solution
|
- Write down the main product of the following reaction: Ethanol underse...
Text Solution
|
- Write the statements of the products (A), (B), (C ), (D), and (E) in t...
Text Solution
|
- Identify (X), (Y), and (Z) in the following synthetic scheme and write...
Text Solution
|
- Compound (A) of molecular formula C(9)H(7)O(2)Cl exists in ketoform an...
Text Solution
|