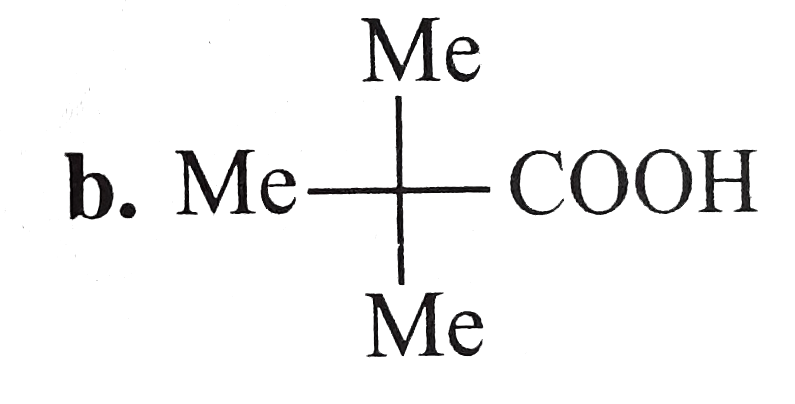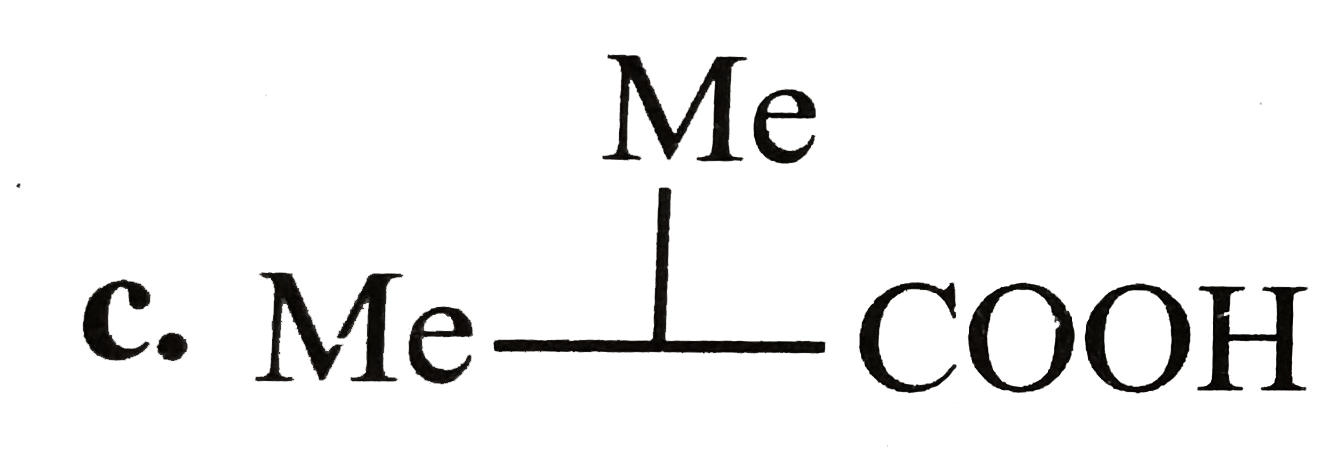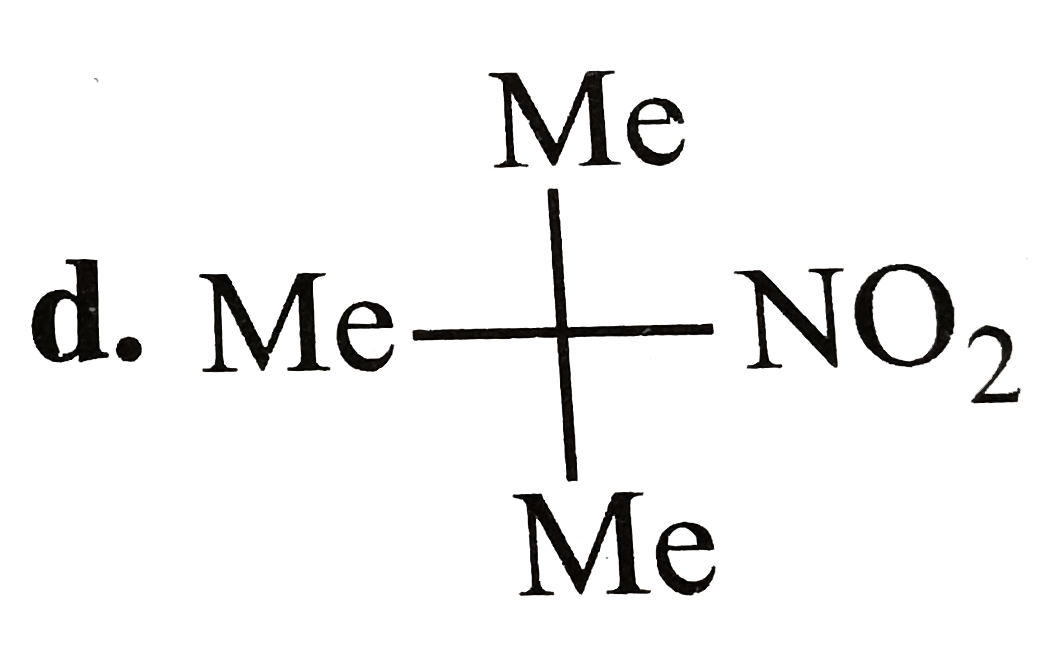A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Assertion And Reasoning)|10 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjective)|26 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|35 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise (Single Correct)
- PhCH(2)CH(2)CH(3) underset((ii) h(2)O)overset((i) CrO(2)Cl(2)//C Cl(4)...
Text Solution
|
- Identify (A) and *(B) in the reaction
Text Solution
|
- The compound Me(3)C-NH(2) on oxidation with acidic KMnO(4) gives:
Text Solution
|
- The product (A) is:
Text Solution
|
- The product (A) and (B) are:
Text Solution
|
- an Organic compound (A) (C(4)H(6)) forms a precipitate with Tollens an...
Text Solution
|
- An alkene on ozonolysis yields only ethanal. There is an isomer of the...
Text Solution
|
- (A) can be:
Text Solution
|
- Which of the following is an incorrect statement?
Text Solution
|
- (A) and (B) are:
Text Solution
|
- The oxidation product of 1,2-cyclopentane diol with HIO(4) or (CH(3)CO...
Text Solution
|
- Chromic anhydride in H(2)SO(4) is not blue by:
Text Solution
|
- Which of the following reactions is correct?
Text Solution
|
- Fehling's solution can make distinction between:
Text Solution
|
- C(6)H(10)O(3) (Keto ester)(A)underset(Delta)overset(NaOH+I(2))(rarr) Y...
Text Solution
|
- Suggest a suitable oxidising reagent for the following conversions:
Text Solution
|
- C(2)H(5)O-overset(O)overset(||)(C )-OC(2)H(5) overset(2MeMgBr)(rarr) (...
Text Solution
|
- Which of the following compounds will not give haloform reaction?
Text Solution
|
- Identify the set from the following which connot form acetone in a sin...
Text Solution
|
- (A) will not
Text Solution
|