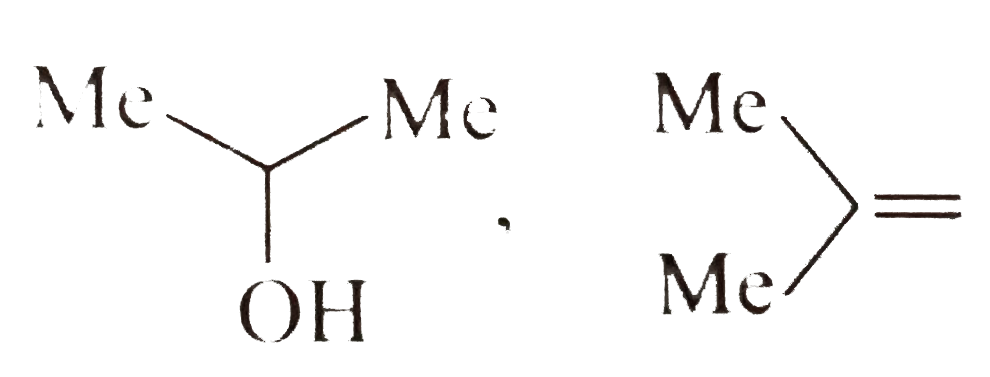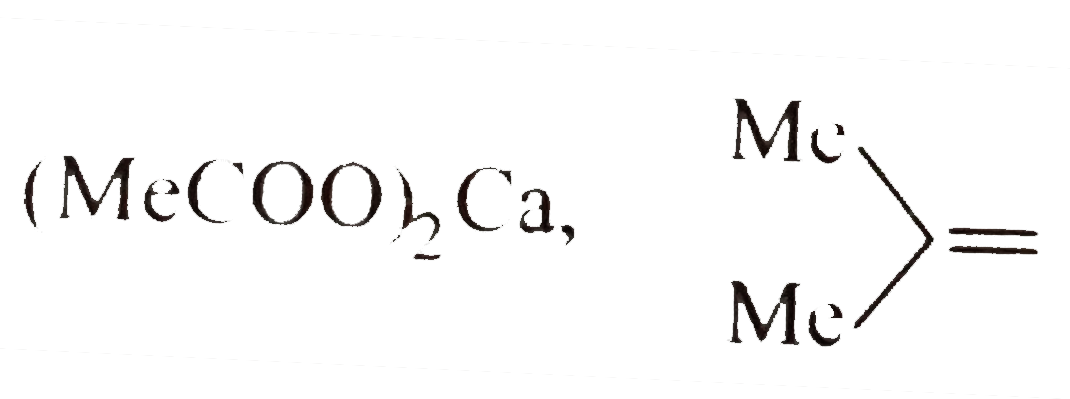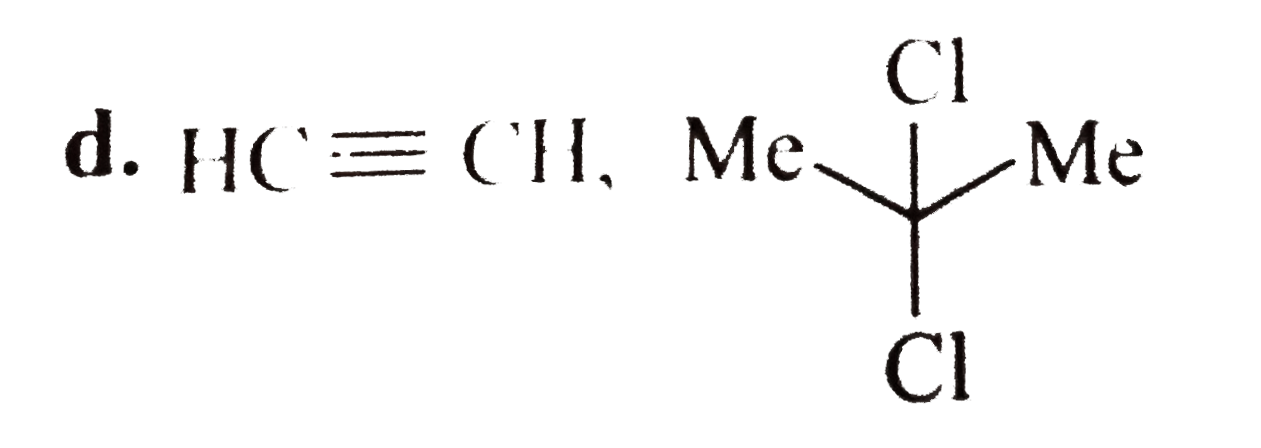A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Assertion And Reasoning)|10 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjective)|26 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|35 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise (Single Correct)
- C(2)H(5)O-overset(O)overset(||)(C )-OC(2)H(5) overset(2MeMgBr)(rarr) (...
Text Solution
|
- Which of the following compounds will not give haloform reaction?
Text Solution
|
- Identify the set from the following which connot form acetone in a sin...
Text Solution
|
- (A) will not
Text Solution
|
- CH(3)CH(2)NO(2) underset(at 0^(@)C)overset(NaNO(2)+HBr)(rarr) (A). (A)...
Text Solution
|
- Which of the following does not give Liebermann's nitroso reaction?
Text Solution
|
- Of the following compounds, whose ozonolysis proves the Kekule structu...
Text Solution
|
- CH(3)-overset(CH(3))overset(|)(CH)-NO(2) underset(at 0^(@)C)overset(Na...
Text Solution
|
- Which of the following compounds are (A), (B), (C ), and (D)? Ph-ove...
Text Solution
|
- The compound (A)
Text Solution
|
- Fenton's regent (Fe^(2+)+H(2)O(2)) with benzene gives:
Text Solution
|
- Lactic acid on oxidation with Fenton's reagent gives:
Text Solution
|
- The compounds (A) and (B) are:
Text Solution
|
- (A) is:
Text Solution
|
- The compound (A) is:
Text Solution
|
- The compound (A) is:
Text Solution
|
- Which single reagent can be used in the following conversions?
Text Solution
|
- Et-N^(o+)-=C^(ɵ) underset(or HgO or O(3))overset(Cl(2)+DMSO)(rarr). Th...
Text Solution
|
- Imines or enamines are selectively reduced to 1^(@) or 2^(@) amines wi...
Text Solution
|
- Caprolactam on reduction with LAH or H(2)+Pt or Pd gives:
Text Solution
|