Select the aromatic, anti-aromatic, and non-aromatic a compounds.
1. ,2. ,3.
4. ,5
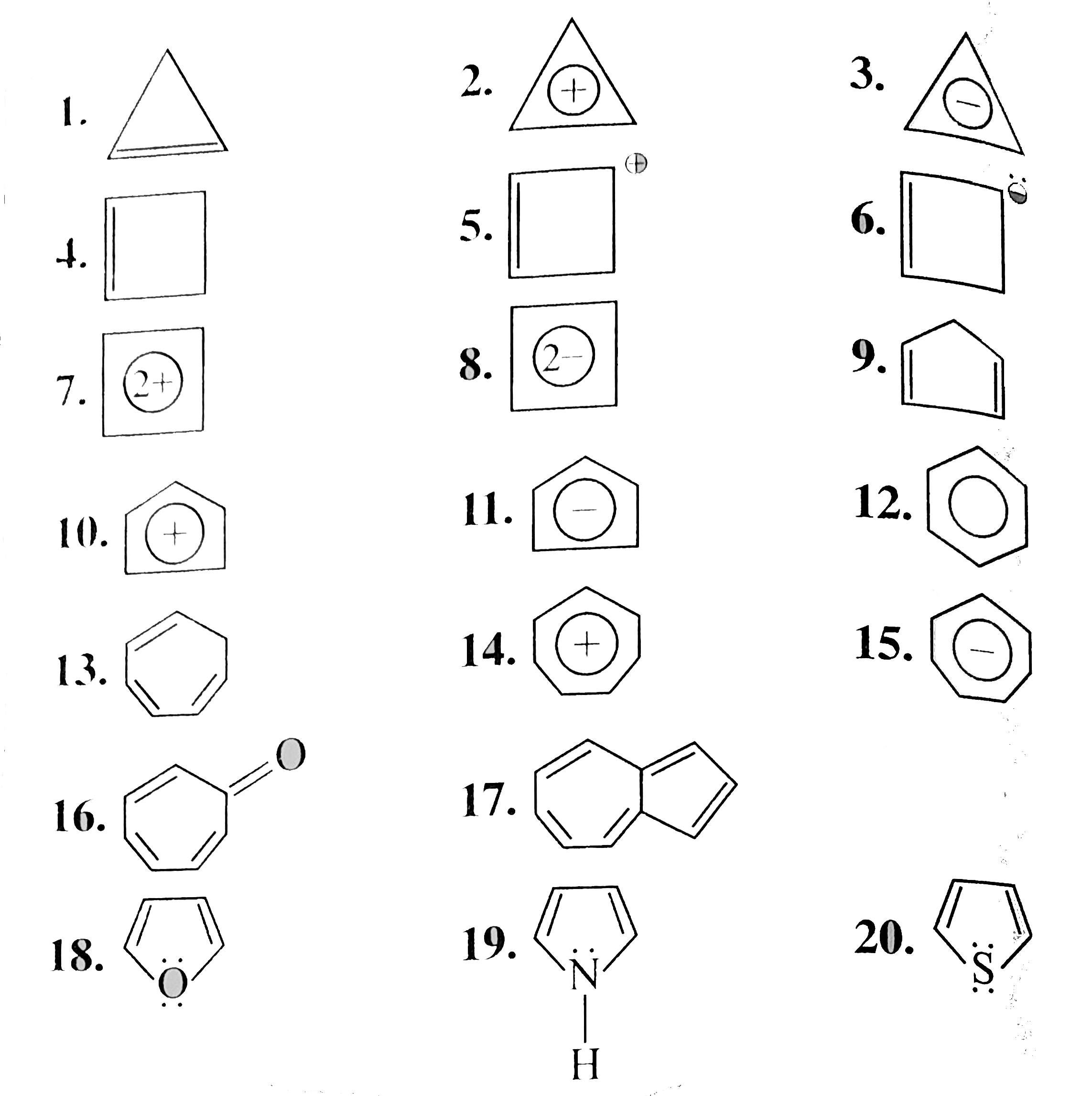
Select the aromatic, anti-aromatic, and non-aromatic a compounds.
1. ,2. ,3.
4. ,5




1. ,2. ,3.
4. ,5




Text Solution
Verified by Experts
1. Non-aromic
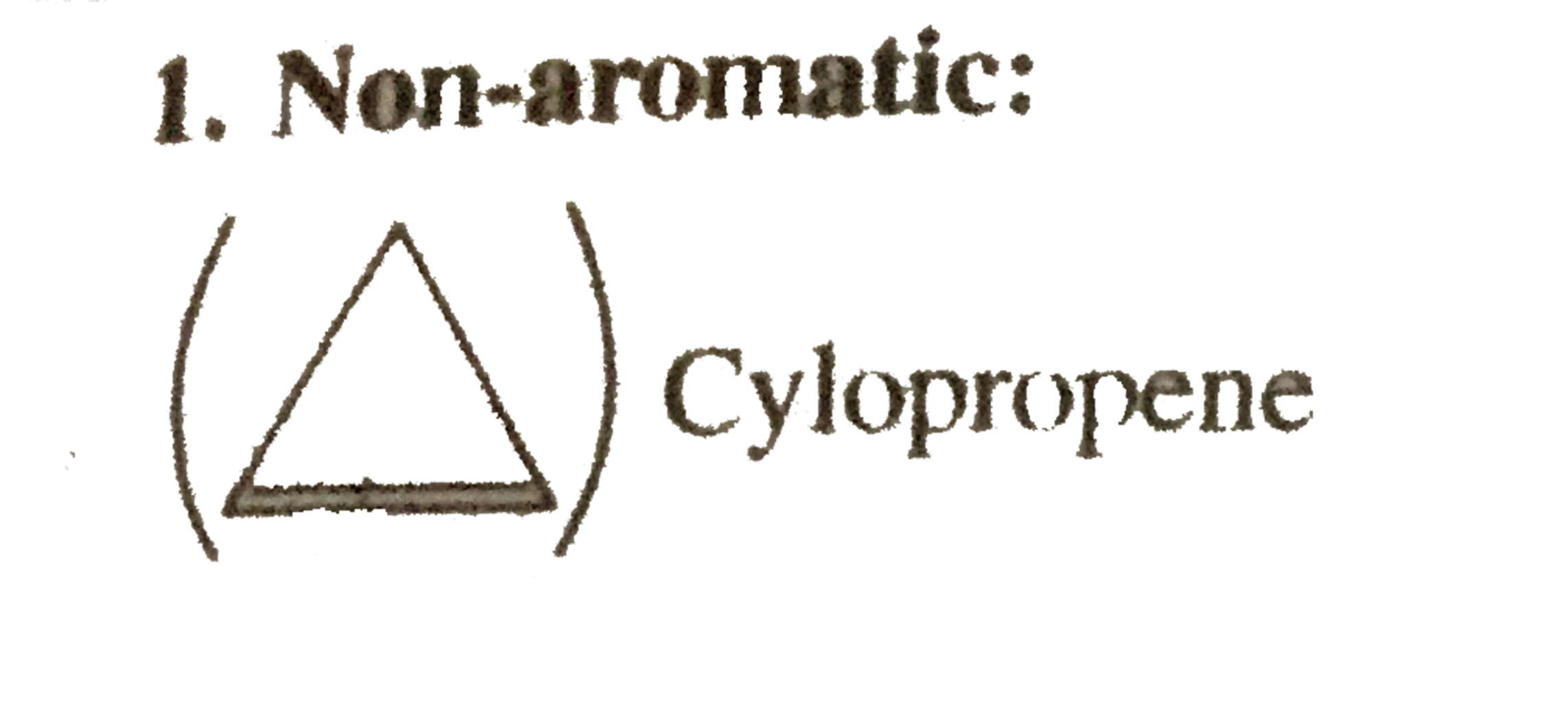 Cylopropene
Cylopropene
Cyclic, planner, `2pi vec(e) s` system, folows `(4pi + 2)pi vec(e)` but not in delocalisation or not in resonance.
2. Aromic:
 Cylopropenyl cation
Cylopropenyl cation
Cylic, planner `2pi vec(e)s` system follow `(4pi + 2) pi vec(e)s` and `2pi vec(e)s` are in delocalisation or in resonance with positive charge.
3. Anti-aromatic:
 Cycloprepenyl anion
Cycloprepenyl anion
Cyclic, planner, `4 vec(e) s` system `(2pi vec(e) + 2 vec(e)` from one negative charge), follows `4n` rule and `2pi vec(e)s` are in conjugatioln with one negative charge and are in delocalisation or in resonance with negative charge.
4. Non-aromatic:
 Explained in Section 11.10 (c)`
Explained in Section 11.10 (c)`
5. Non-aromatic:
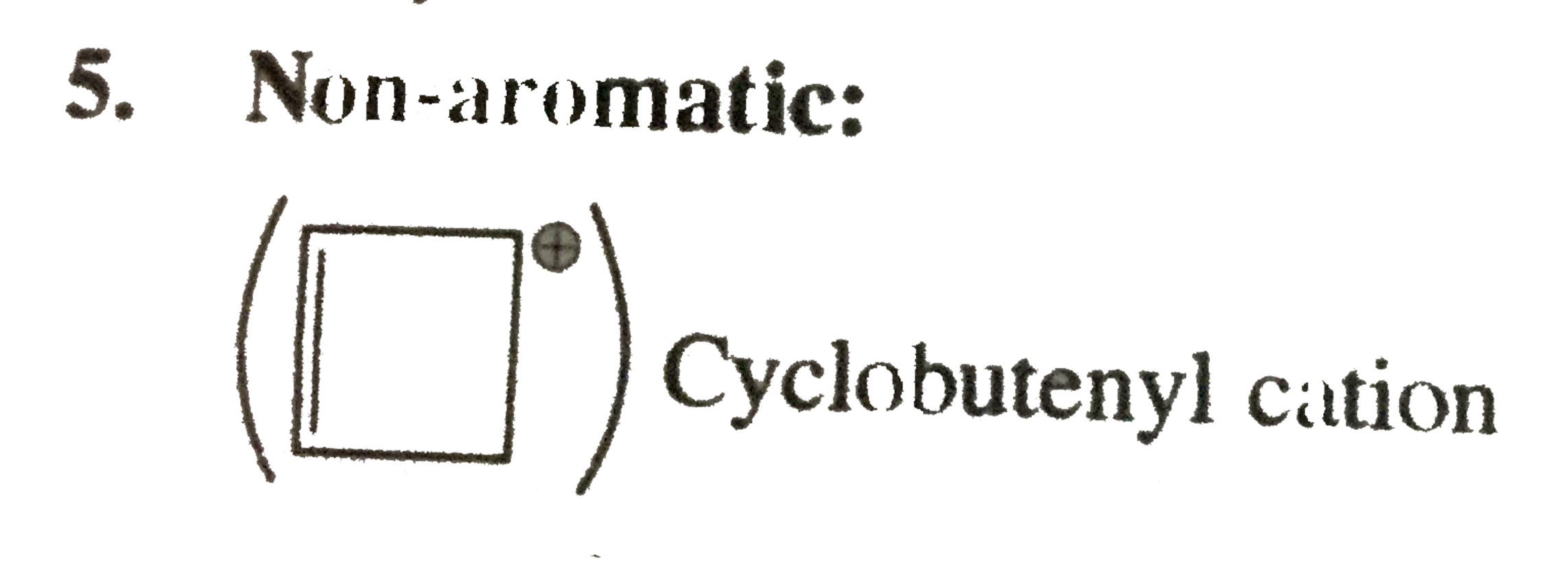 Cyclobutenyl cation
Cyclobutenyl cation
Cyclic, plannar, `2pi vec(e)s` system, follows `(4n + 2) pi vec(e) s, (pi = 0)`, but `2pi vec(e)s` are not in complete delocation with positive charge.
6. Non-aromatic:
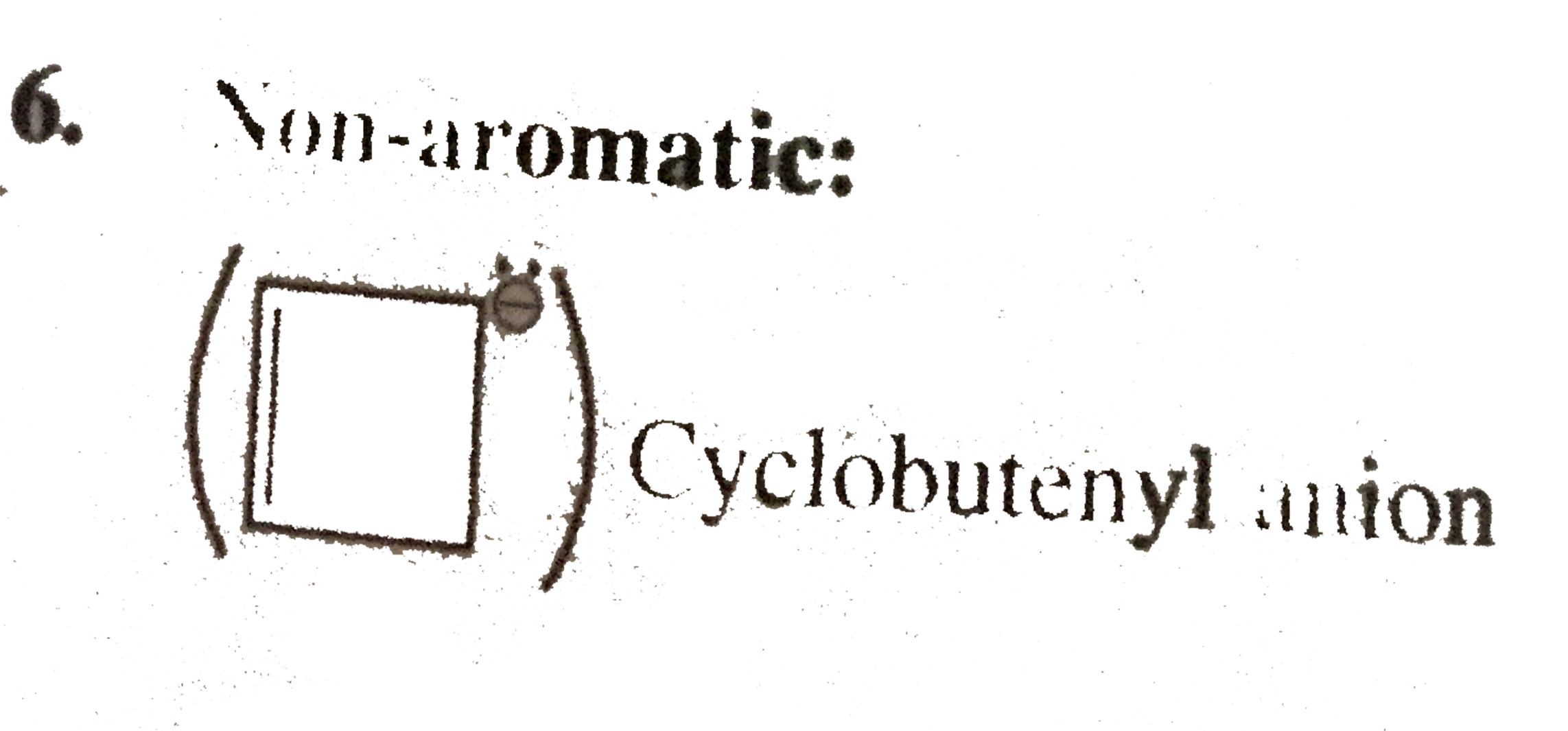 Cyclobutenyl anion
Cyclobutenyl anion
Cyclic,n planar, `4pi vec(e)'s` system `(2pi vec(e) + 2 vec(e)` from one negative charge ) follows `4n` rule `(n = 1)` but `2pi vec(e)'s` are not in complete delocalisation with negative charge.
7. Aromatic:
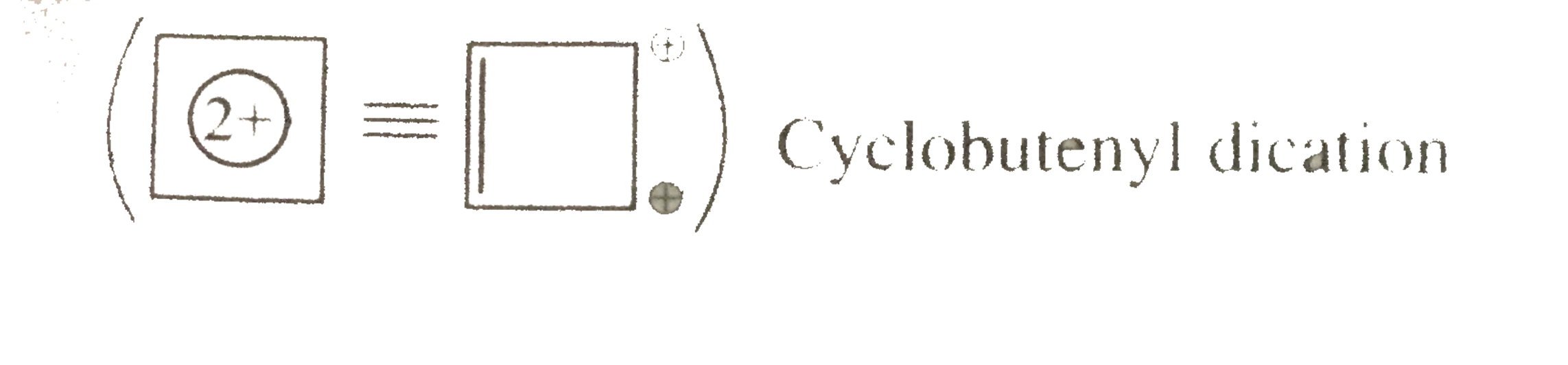 Cyclobutenyl direction
Cyclobutenyl direction
Cylic, planar `@pibar(e)` 's system, following `(4n+2)` rule `(n=0)` , and `2pibar(e)` 's are in complete delocalisation or in resonance with two positive charges.
8. Aromaitc: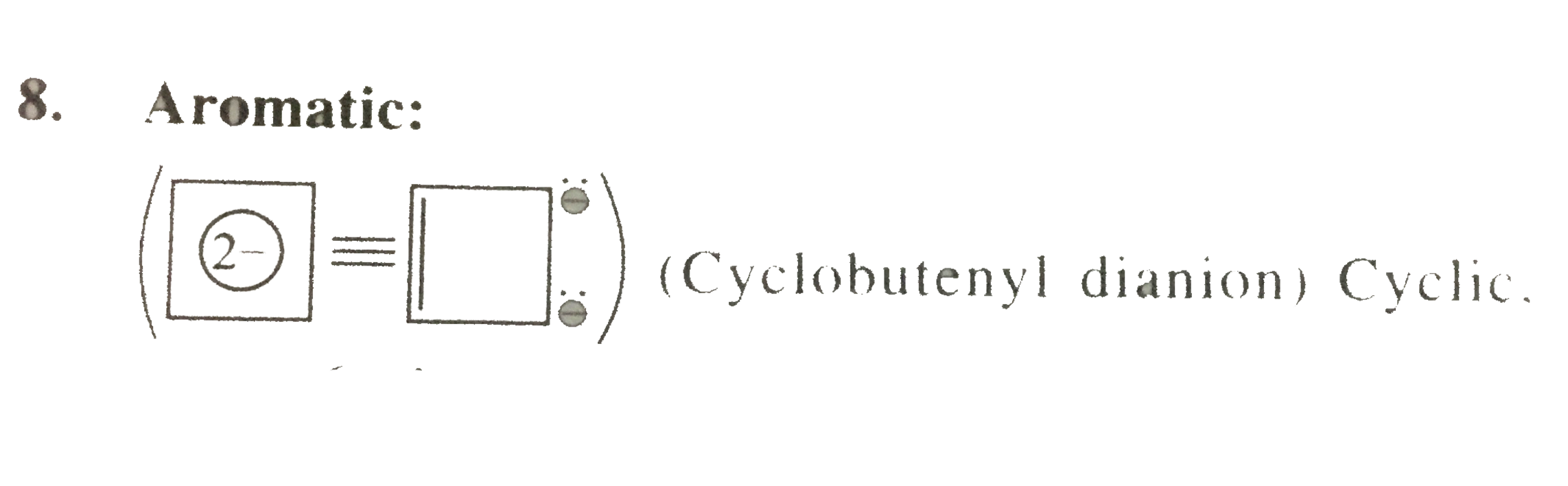 (Cyclobutenyl dianion) Cyclic, planar `6bar(e)` 's system `[2pibar(e)+4bar(e)` 's (from two negative charges) ] follows `(4n+2)` rule `(n=1)` ansd 2pibae(e)` 's are in negitive charges.
(Cyclobutenyl dianion) Cyclic, planar `6bar(e)` 's system `[2pibar(e)+4bar(e)` 's (from two negative charges) ] follows `(4n+2)` rule `(n=1)` ansd 2pibae(e)` 's are in negitive charges.
9. Non-aromatic: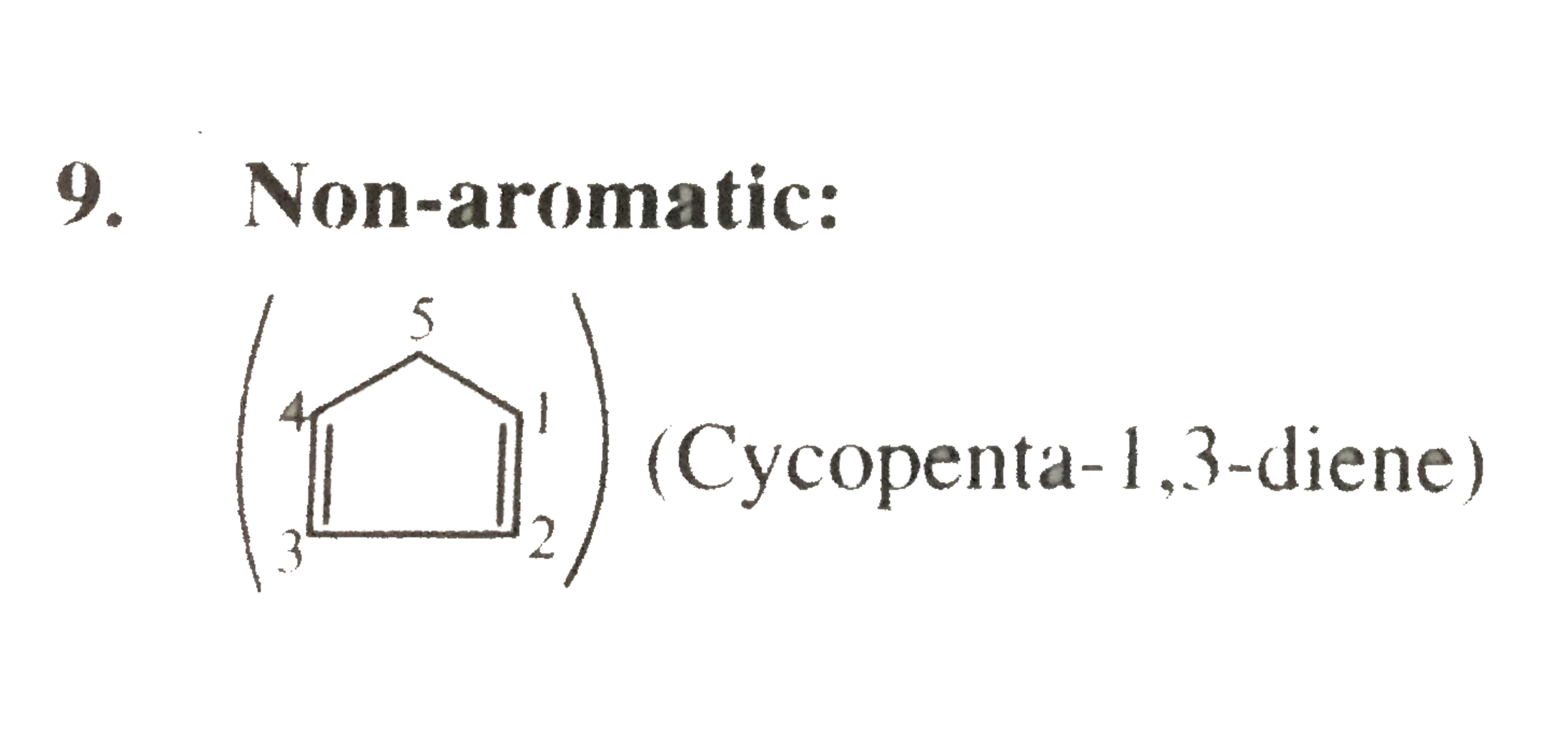 (Cycopenta `-1,3-` diene)
(Cycopenta `-1,3-` diene)
Cyclic, planar `4pibar(e)` 's system, follows `4n `rule `(n=1)` ,. and `4pibar(e)` 's are mot in complete deloalisation or not in resonance.
10. Antiaromatic: (Cyclopenta `-1,3-` dienyl cation)
(Cyclopenta `-1,3-` dienyl cation)
Cylic, planar, `4pibar(e)` 's system, follows `4n` rule `(n=1)` , and `4pibar(e)` 's are in complete delocalisation or in resonance with positive charge.
11. Aromatic: (Cyclopentea `-1,3-` dienyl anion)
(Cyclopentea `-1,3-` dienyl anion)
Cycilc, planar `6bar(e)` 's system `(4pibar(e)` 's+2bar(e)` 's from one negative charge) follows `(4n+2)` rule `(n=1)` , and `4pibar(e)` 's are in complete deloclisation or in resonanace with negative charge.
12. Aromatic: (Benzene)
(Benzene)
Cyclic, Planar, `6pibar(e)` 's system, follows `(4n+2)` rule `(n=1)` , `6pibar(e)` 's are in complete delocalisation or in resonace.
13. Non-aromatic: (Cyclohepta `-1,3,5-` triene)
(Cyclohepta `-1,3,5-` triene)
Cyclic planar, `6pibar(e)` 's system, follows `(4n+2)` rule `(n=1)` , but `6pibar(e)` 's are not in complete delocalisation or not in resonance.
14. Aromatic:
 Cyclohepta `-1,3,5-` trienly cation
Cyclohepta `-1,3,5-` trienly cation
Cyclic, planar `6pibar(e)` 's system, follows `(4n+2)` rule, `(n=1)` , and `6pibar(e)` are in complete delocalisation or in resonance with positve charge.
15. Cyclohepta `-1,3,5-` trienly anion
Cyclohepta `-1,3,5-` trienly anion
Cyclic, planner `8vec(e) s` system (`6pi vec(e) + 2 vec(e)` from one negative charge) follows `4n` rule `(n = 2)`, and `6pi vec(e) s` are in complete delocalisation or in resonanance wtih negative charge.
16. Aromatic:
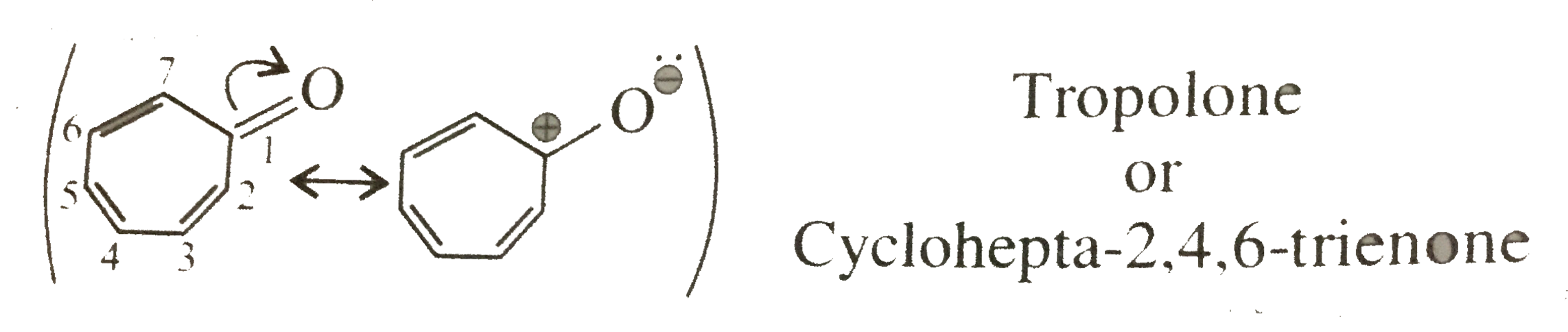
Cyclic, planner, `6pi, vec(e) s` of the ring, follows `(4pi + 2)` rule `(n = 1)` and `6pi vec(e)` are in complete delocalisation or in resonance with positive charge on the ring.
17. Aromatice:
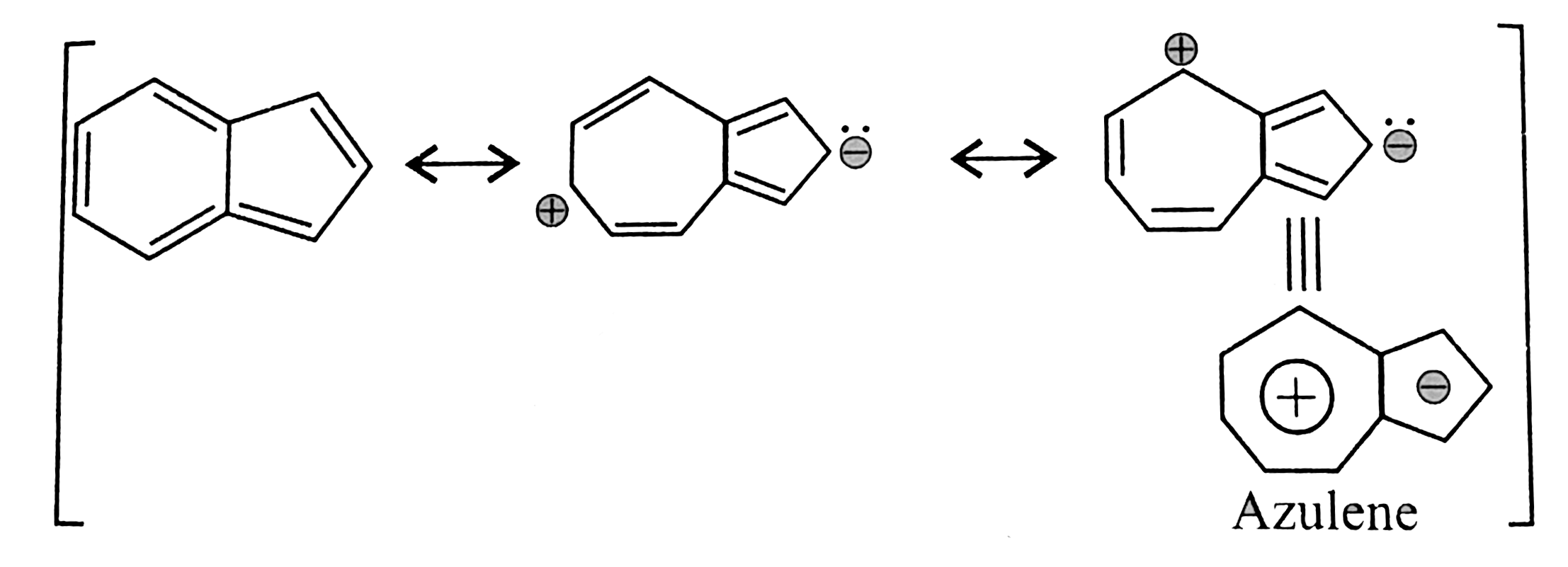
Neither cyclophertarience nor cycloperntadience alone is aromatic but resonanace structure bears a positive charge in the secen-membered ring, given it an aromatic character of cycloherpratrientyl cation, while the five-membered ring bears a negative charge making it similar to the aromatic cyclopentadienty1 atom cyclopentadiency a anion. This cahrge sepration is reponsible for the observed dipole moment. Moreover, it is cyclic, planner with `10pi vec(e)s` are in complete decolaiation or in resonance.
18. Aromatic:
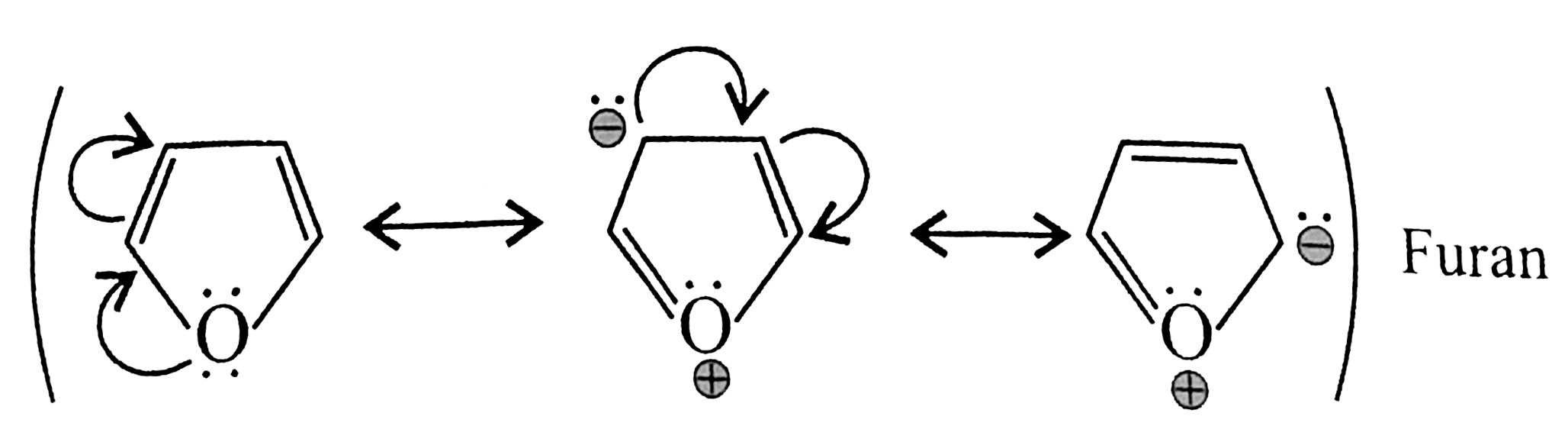
Cyclic planner, `6 vec(e)s` system (`4pi vec(e)'s + 2 vec(e)` from only one `I.P`) (only lone pair is required for delocalisation). Follows `(4pi + 2)` rule `(n = 2), 4pi vec(e)s` and `2 vec(e)'s` from `Lp` are in complete delocalistaion or in resonance hence aromatic.
19. Aromatic:
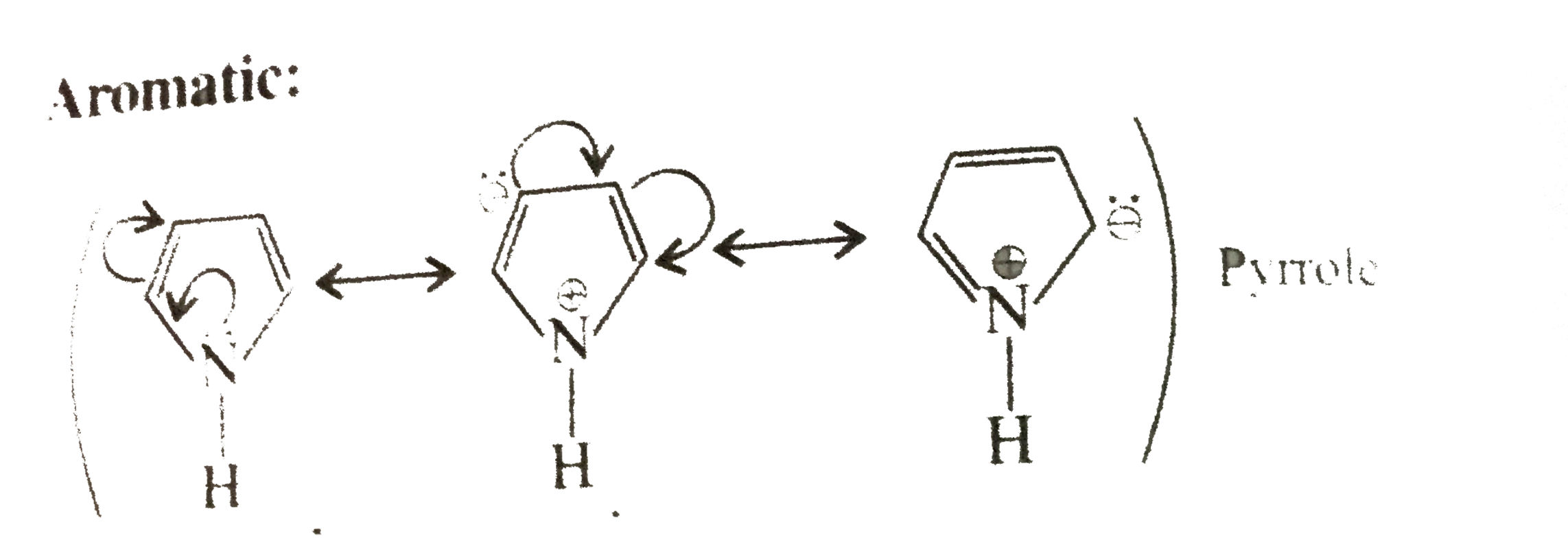
Same explanation as in18.
20. Aromatic:
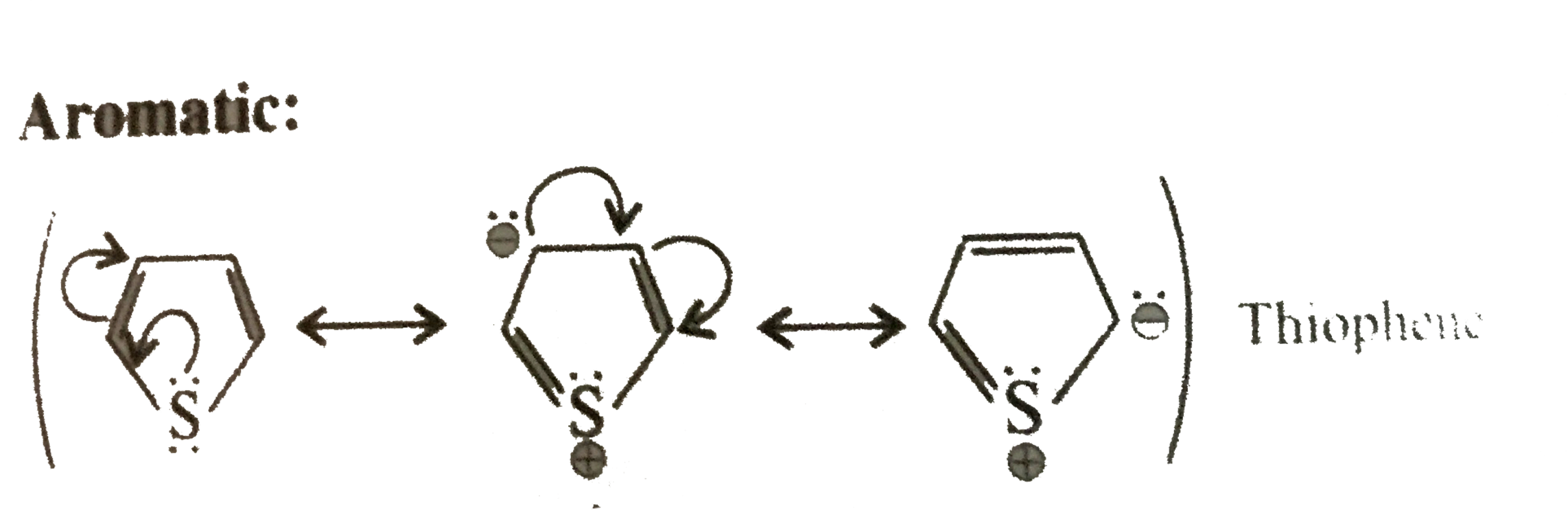
Same explanation as in 18.
21. Aromatic:
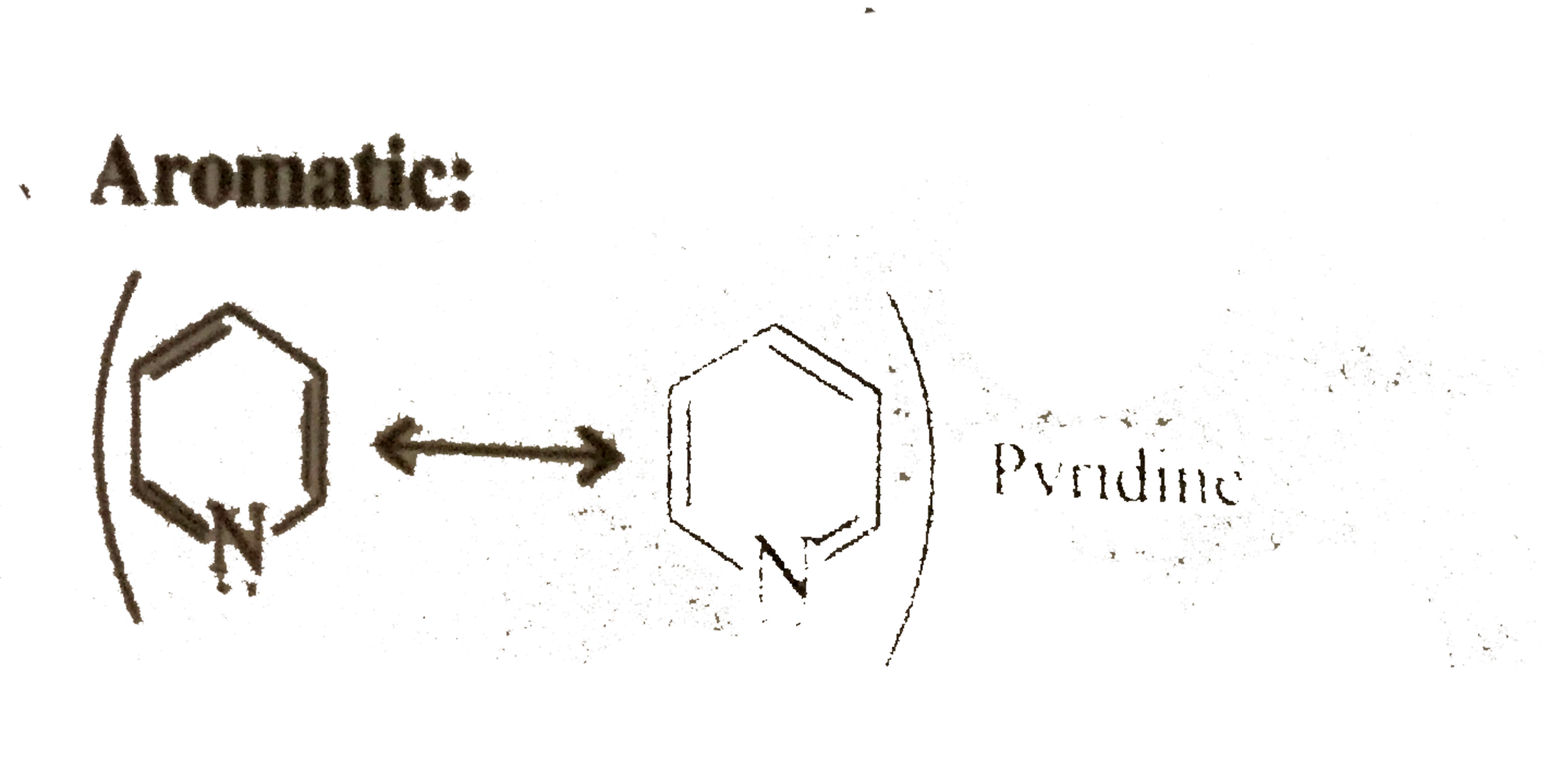
(Lone pair `vec(e)'s` are not used in delocalisation)
Same explanation as in 18.
22. Aromatic:
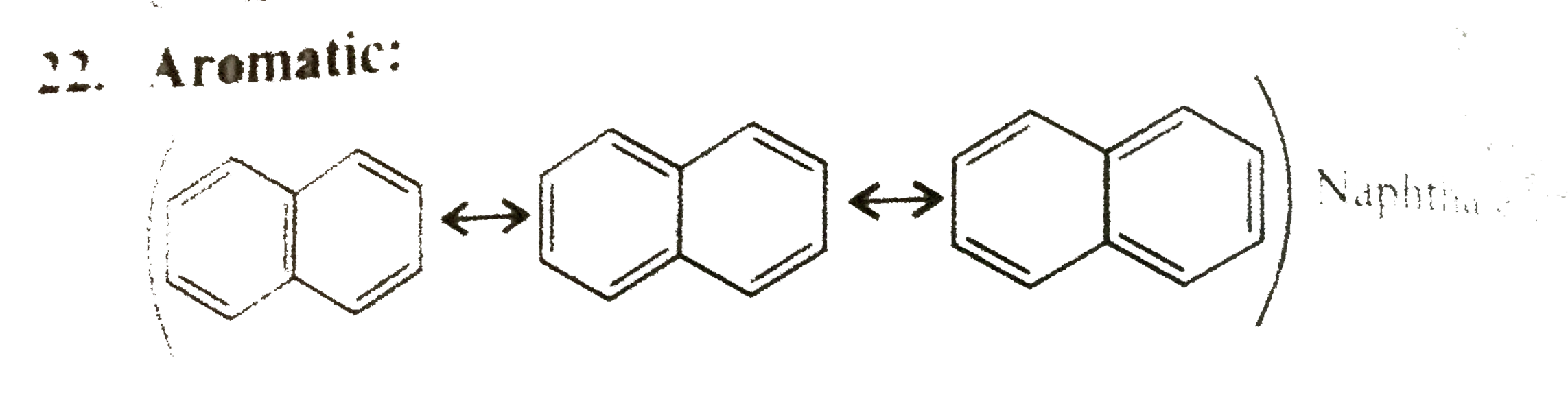
Cylclic, planner, `10pi vec(e)'s` system, follows `(4n + 2)` rule `(n + 2)` in complete delocalisation or in resonance
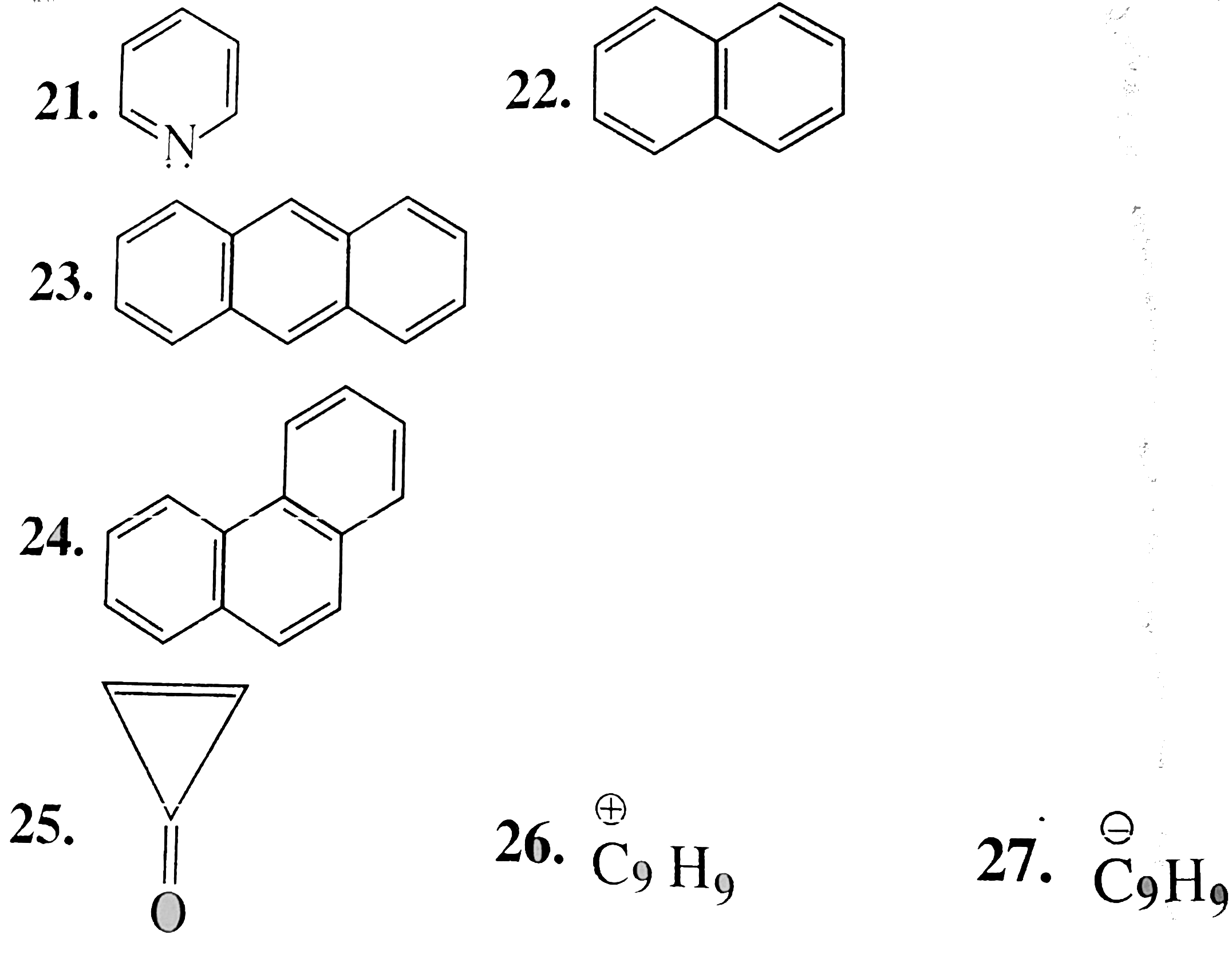
23. Aromatic:
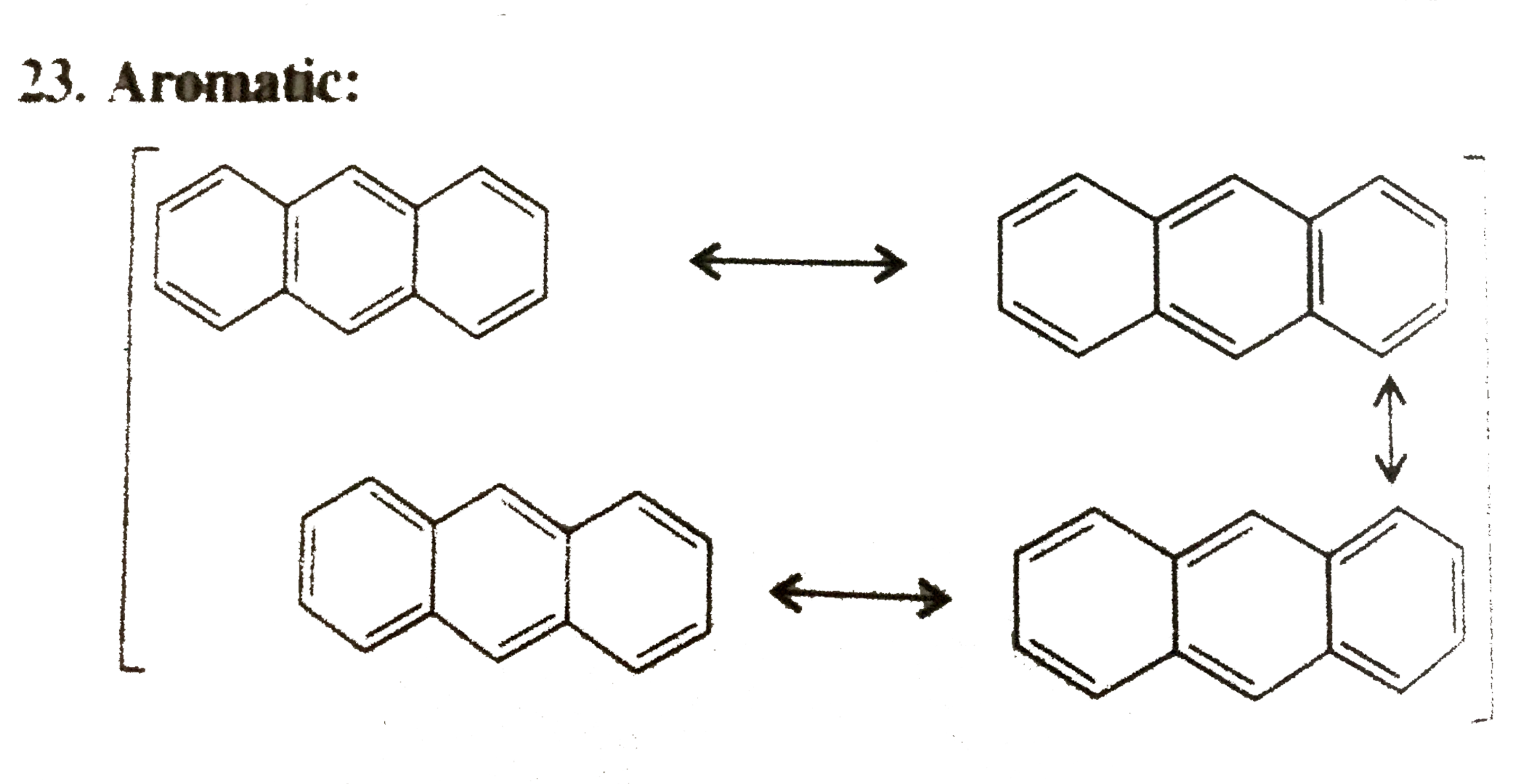
Cylic, planner, `14pi vec(e)'s` system follows `(4n + 2)` rule `(n = 3)`, in complete delocalisiton or resosnance.
24. Aromatic:

Same explanation as in 23. Cyclic palnner, `2pi vec(e)'s` of the ring in complete delocalisation or in resonance with positive charge on the ring, follows `(4n + 2)` rule `(n = 0)`.
26. Anti-aromatic `(overset(o+)(C)_(9) H_(9)):
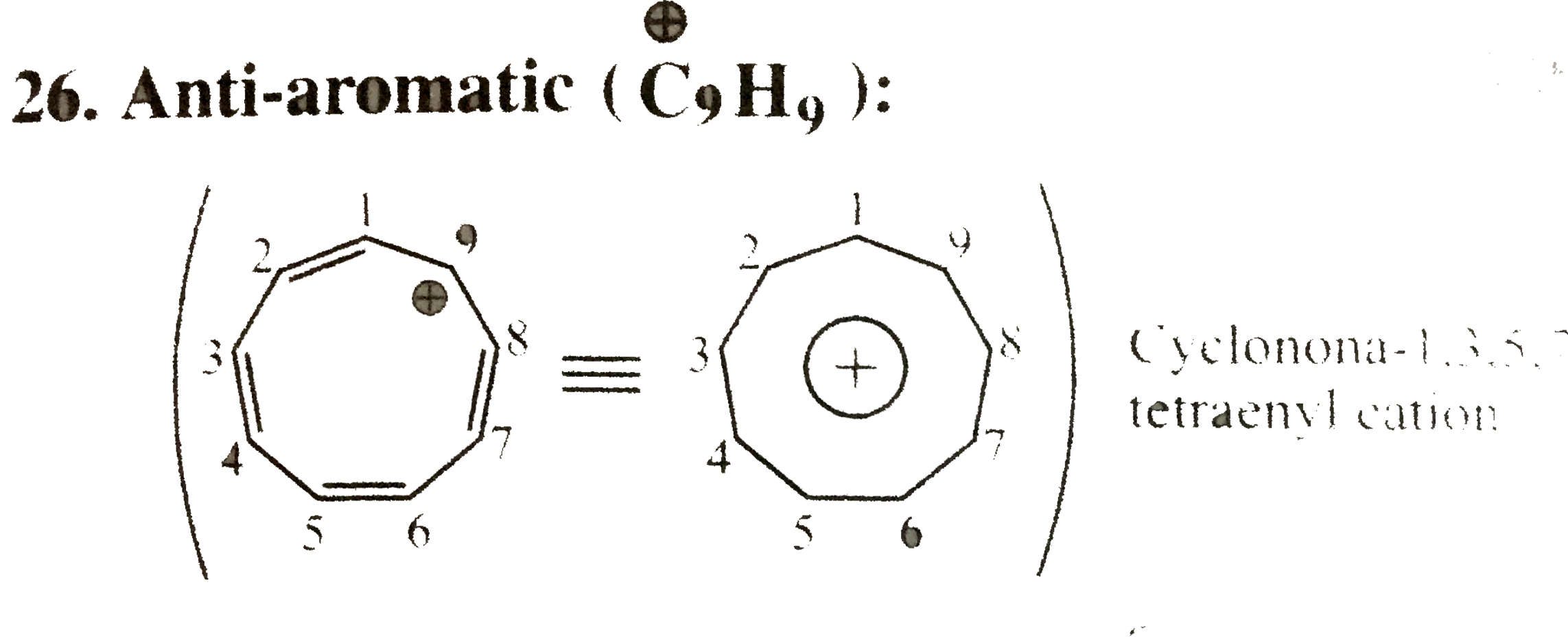
Cyclic, planar, `8pi vec(e)'s` system follows `4n` rule `(n = 2)` and `8pi vec(e)'s are in complete delocalisation or in resonance with positive charge.
27. Aromic`(overset(o-)(C)_(9) H_(9))`:
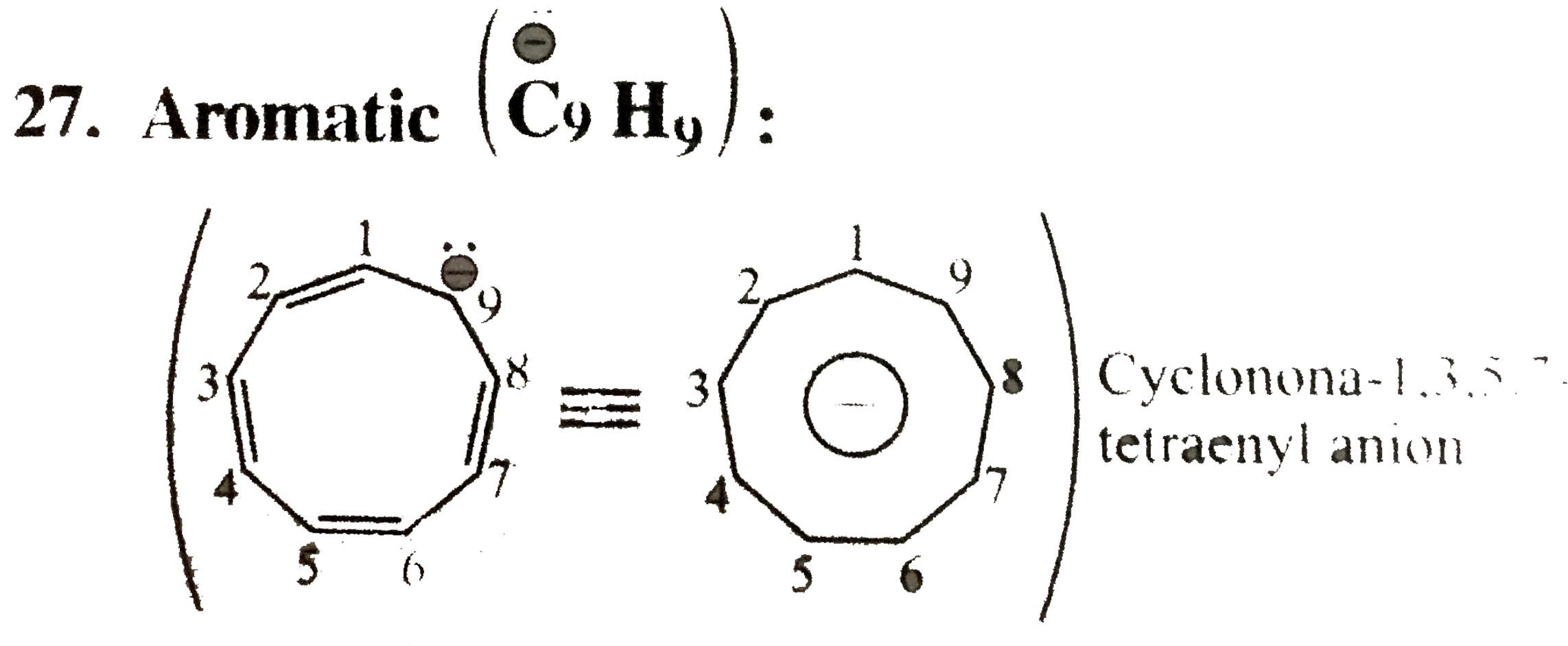
Cyclic planar, `10 vec(e)'s` system `[8pi vec(e)'s + 2 vec(e)` from one negativ charge)], follows `(4n + 2)` rule `(n = 2)`, and `8pi vec(e)'s` are in complate delocalisation or in resonance with negative charge.
28. Non-aromatic `(C_(9) H_(10))`:
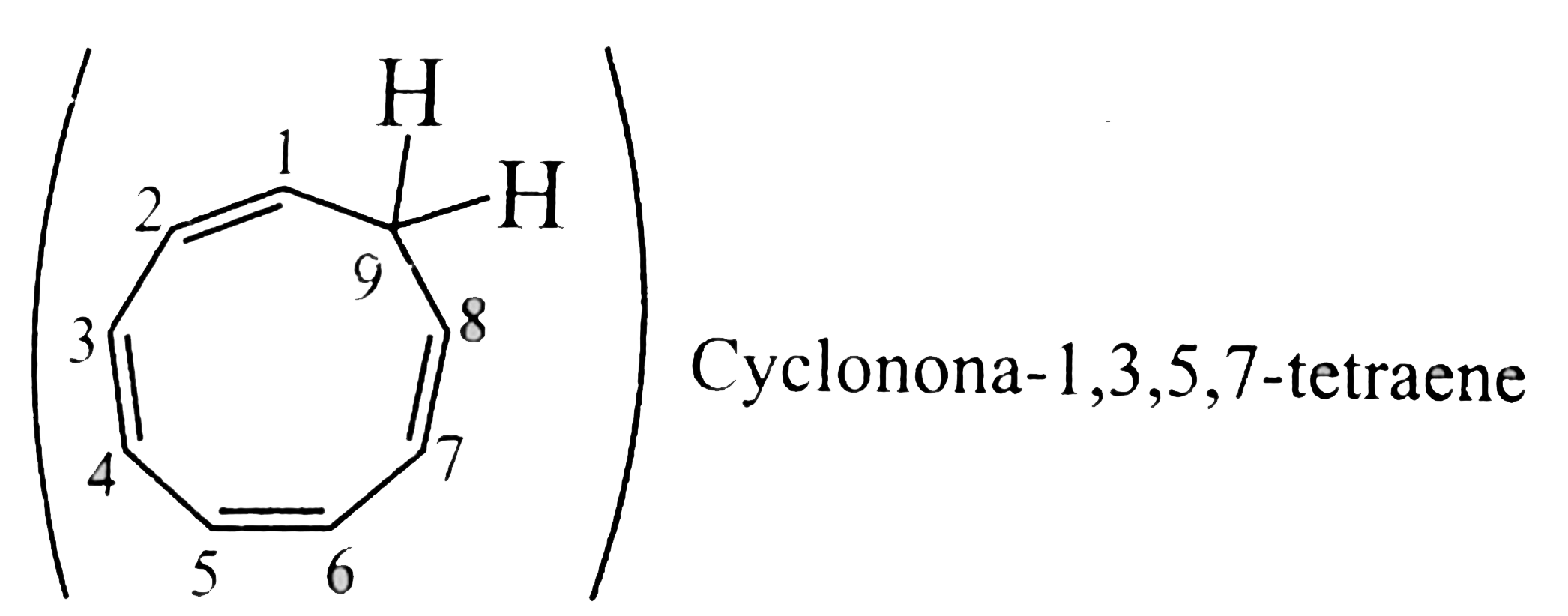
Cyclic, planar, `8pi vec(e)'s` system, follows `4n` rule `(n = 2)`, but tehy are not in complete delocalisationor not in resonance.
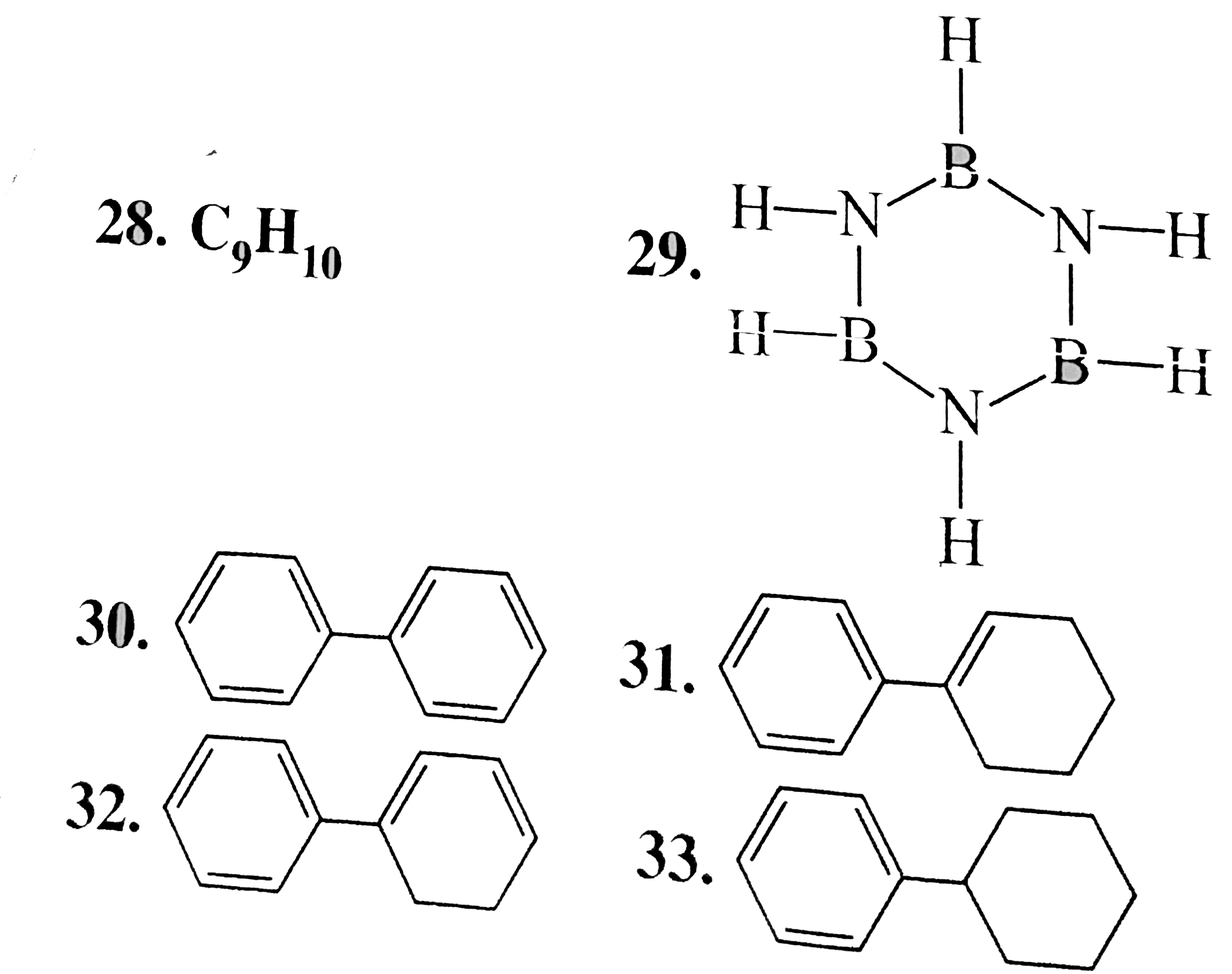
29. Aromatic:
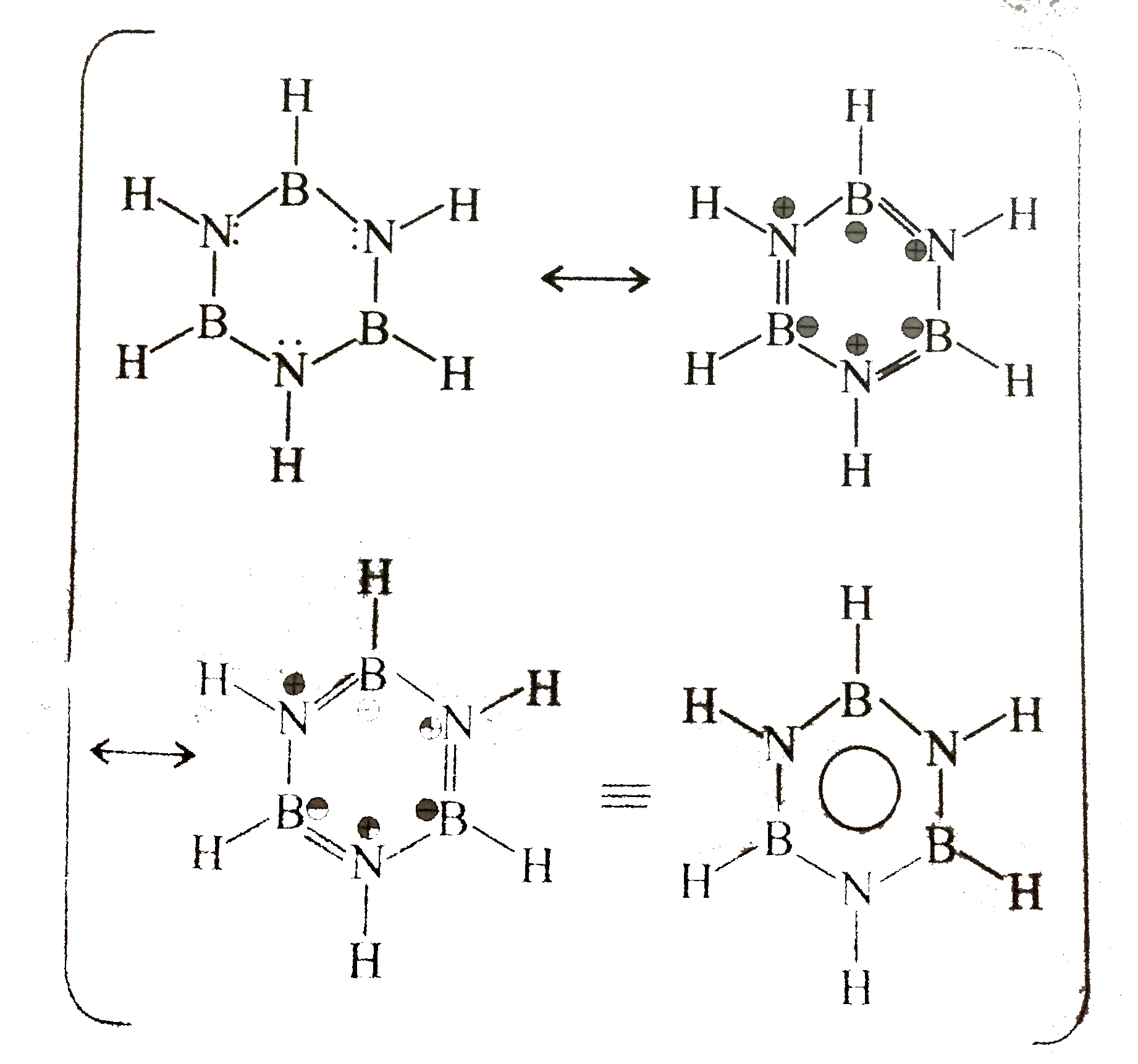
Cyclic, planner, both `N` and `B` are `sp^(2) - hybridised. Each `N` has two bonding `vec(e)'s (LP vec(e)'s)` in a `P`- orbital and each `B` has an emply `p`-orbital, giving a total of six' delocalises, `pi vec(e)'s`, hence aromatic, All `(B-N)` bond lenghths are equal an all ring angles are `120^(@)`.
30. Aromatic: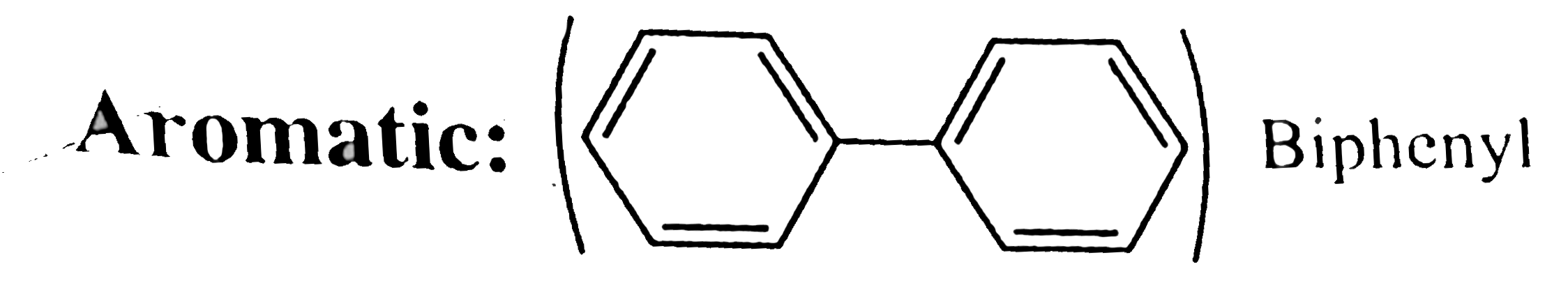
In isolated benene rings, `pi vec(e)'s` are counted seperately in each ring unlike the fused rings, in whcih total `pi vec(e)'s` are counted to verify `(4n + 2)` or `4n` rule.
In biphenyl, each ring has six `pi vec(e)'s` in delocalsation, hence aromatic.
31. Aromatic: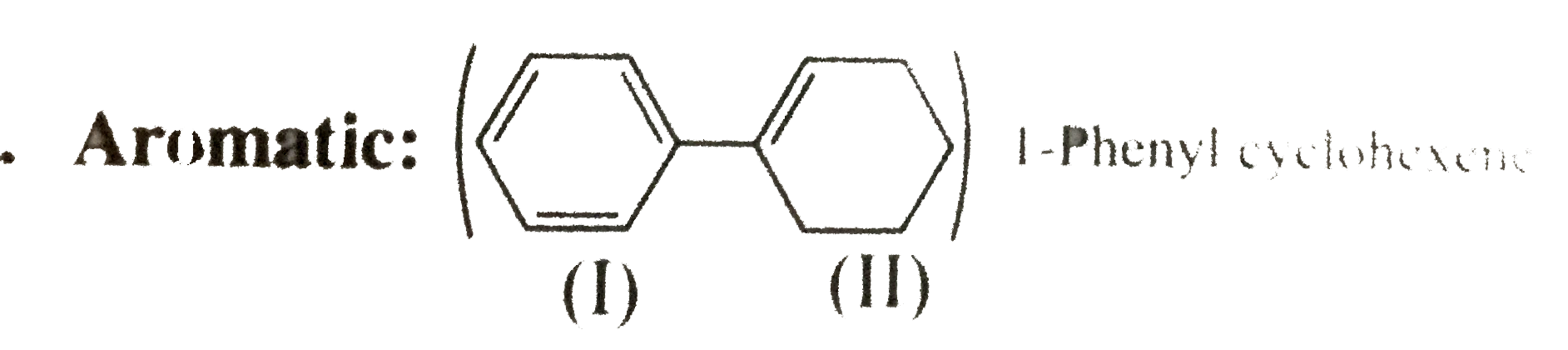
Ring (I) has six `pi vec(e)'s` in delocalisation and is aromatic. But rign (II) has only two `pi vec(e)'s` but not in delocalisation, so ring (II) is non-aromatic. In isolated ring. If one ring is aromatic the compound is said to be aromatic.
32. Aromatic:
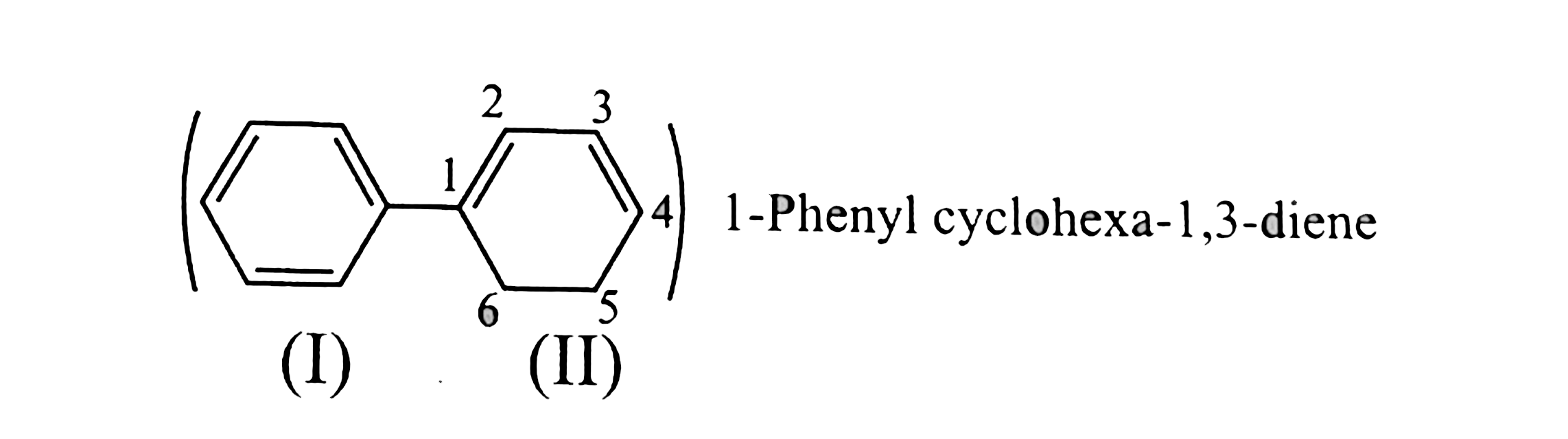
Ring (1) has six `pi vec(e)'s` in deloclisationand is aromatic. But ring (ii) has only four `pi vec(e)'s` but not is delocalisation and is non-armatic. But at least one ring is aromatic, so the compound is armotic.
33. Aromatic: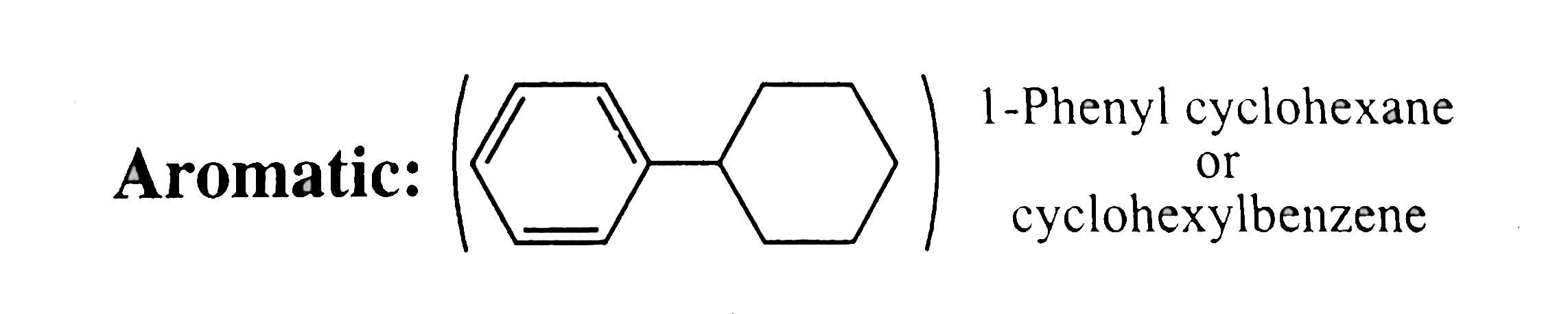
same explanation as in `30-32`.
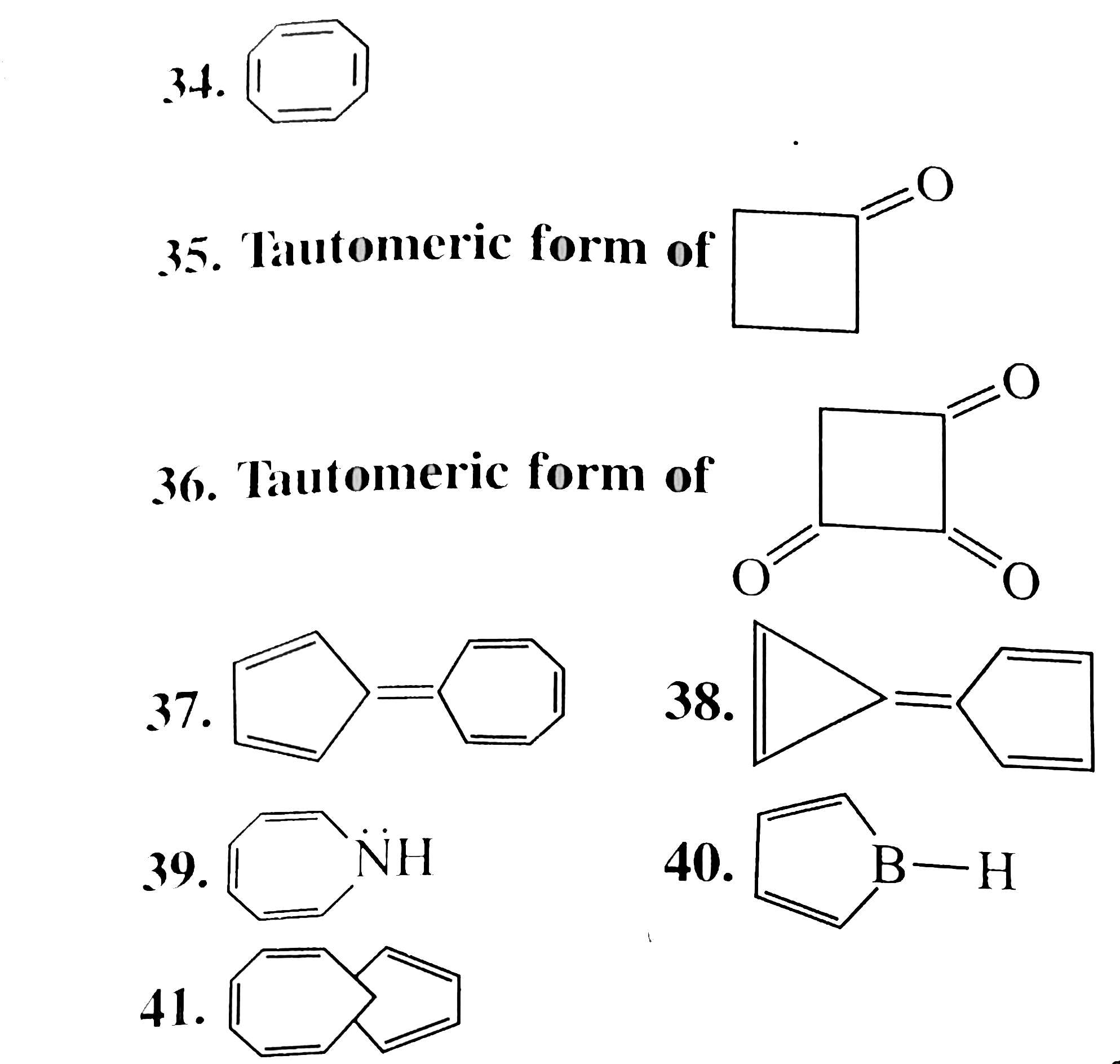
34. Non-armatic:
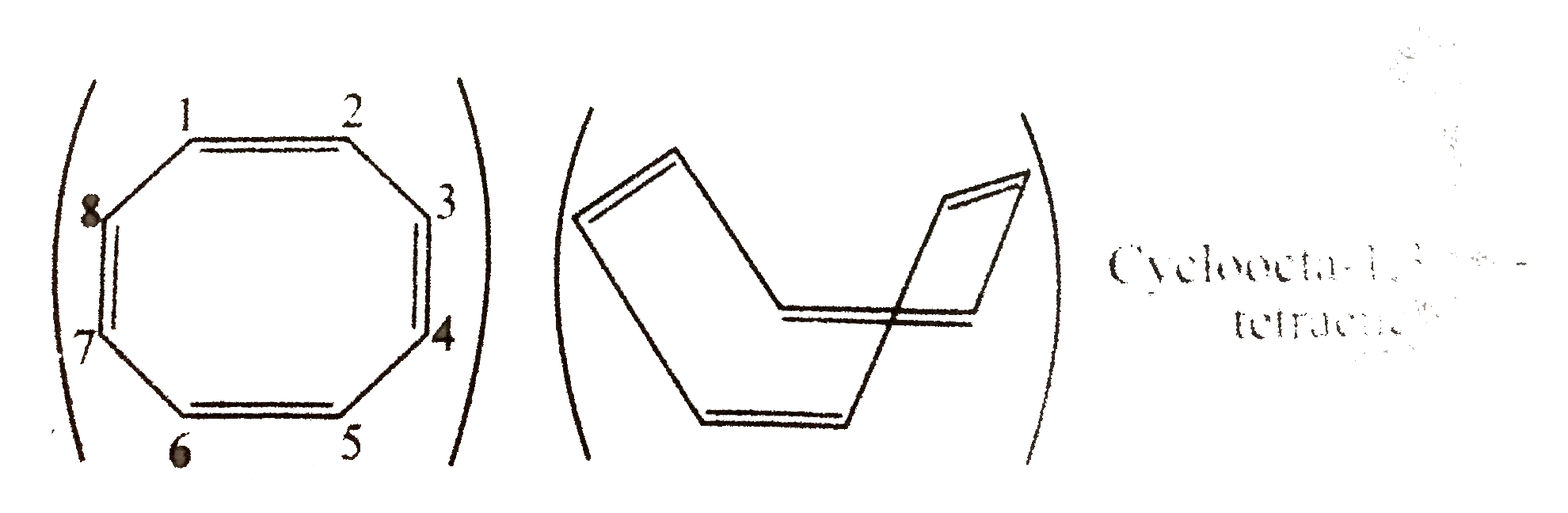
It is not planer but tub-shaped. The `p`-orbits of one `C = C)` are not coplaner with those of a negihbouring `(C = C)` and there can be no effective overlap for delocalisation. The `8pi vec(e)'s` system follows `4n` rule `(n = 2)`, but due to non-planar, it is not anti-aromonic.
35. Anti-aromatic:
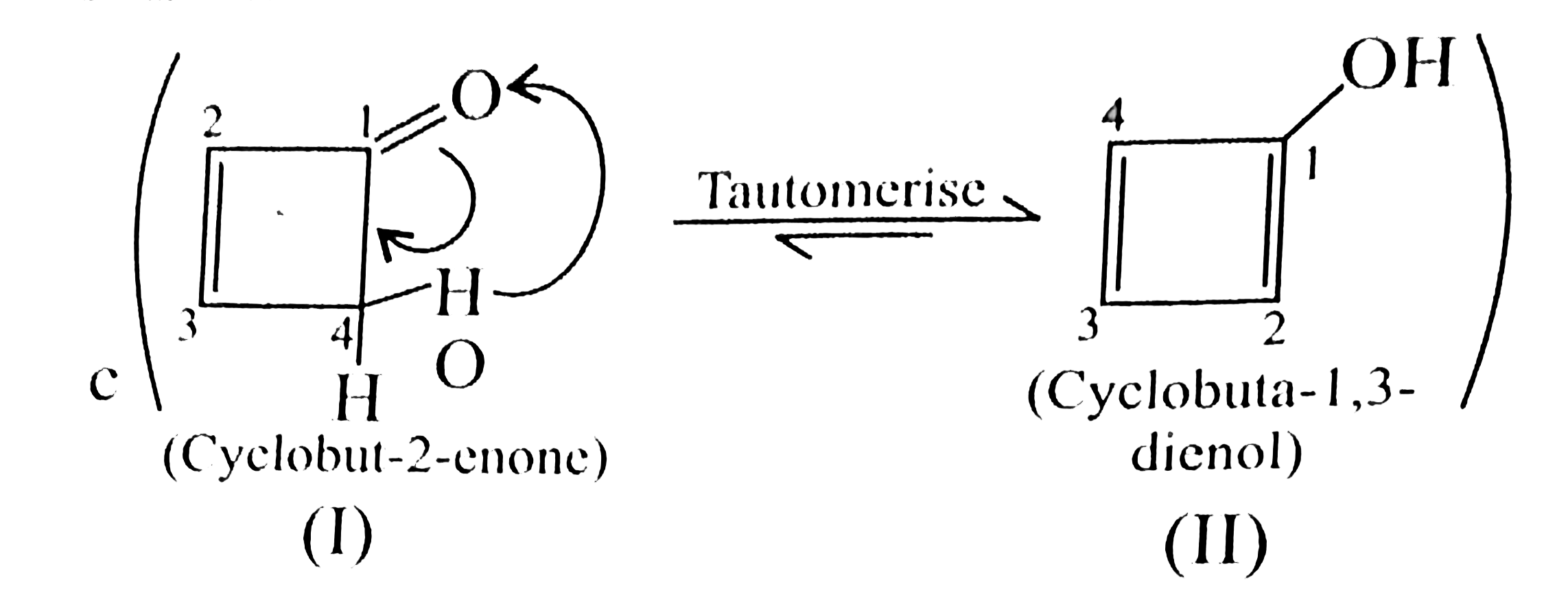
(II) is `4pi vec(e)'s` system in conjugation with complete delocalisation, follows `4n` rule `(n = 1)`, adn is anti-aromatic.
36. Aromatic:
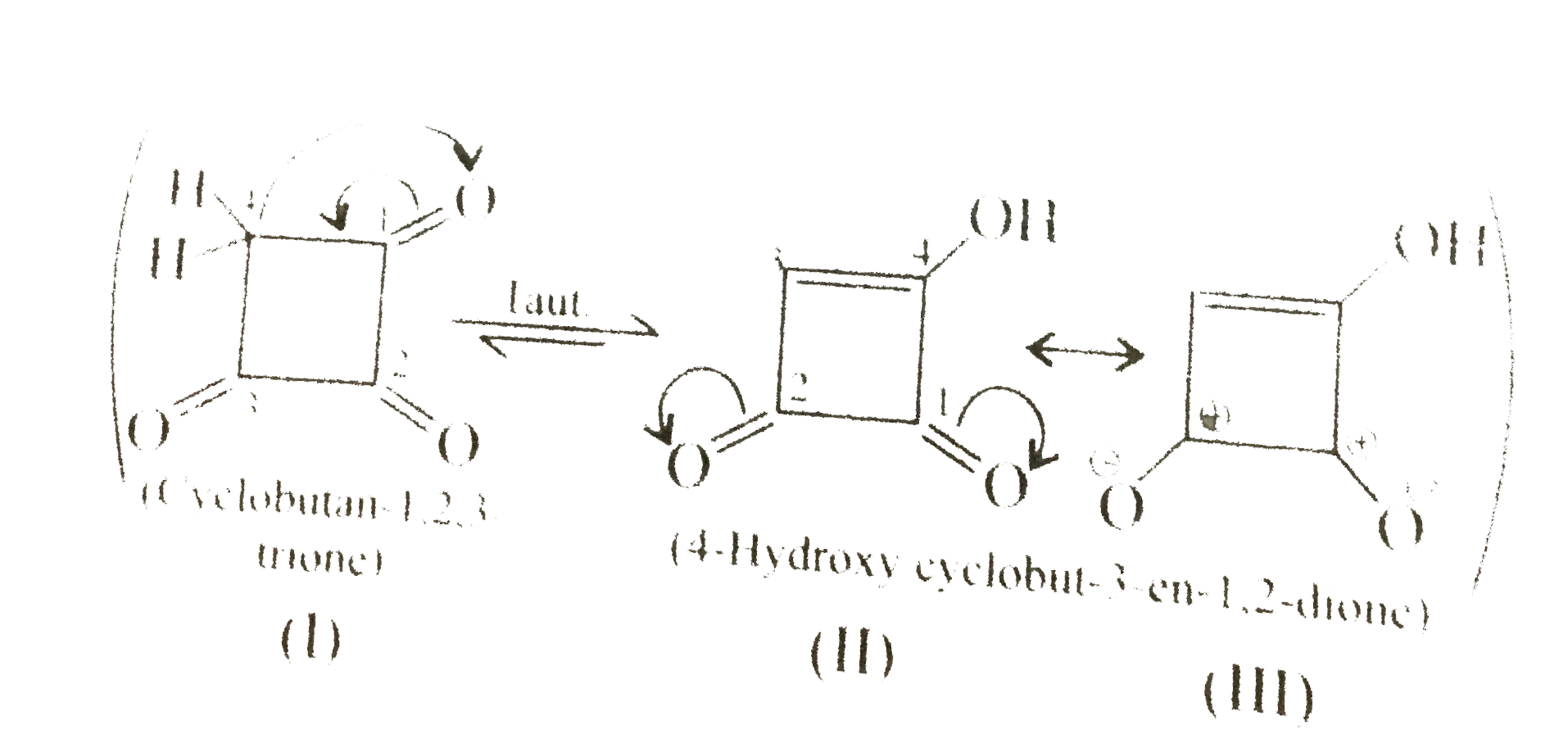
(III) is cyclic, plannar, `2pi vec(e)'s` system and in complete delocalisation wtih two positive charges on the ring (similar to cyclobutenyl dication). It follows `(4n + 2)` rule `(n = 0)`.
37. Aromactic:
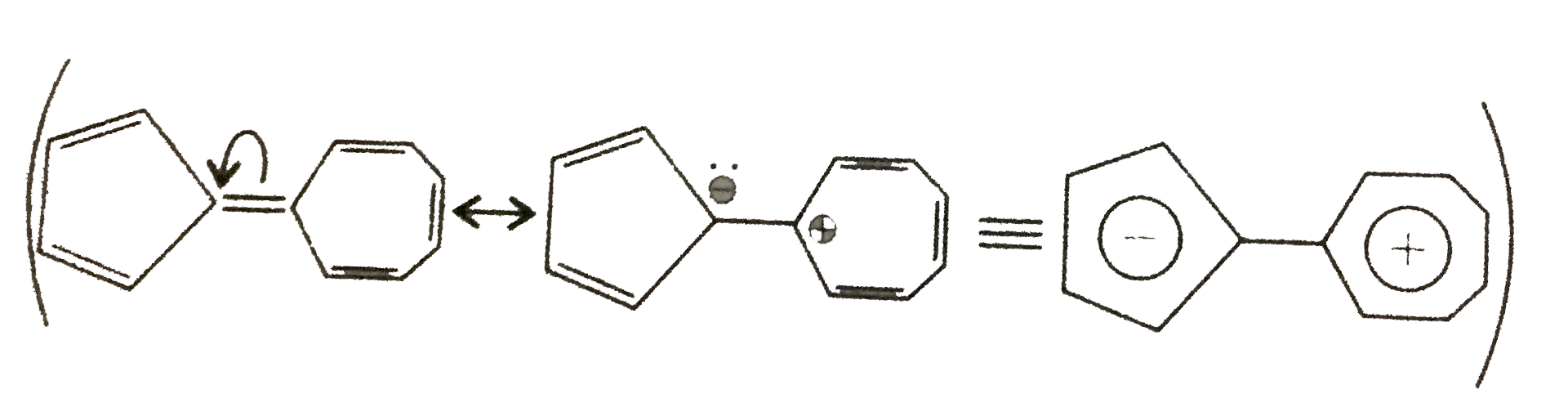
Neither the five-membered not the seven-membered ring alone is aromatic character of cycloheptatrieny' cation, while the five-membered ring bears a negative charge making it similar to the aromatic cyclopentadienyl anion. This charge separation is reponsible for the observed dipole moment. Further, each ring is aromatic.
38. Aromatic:
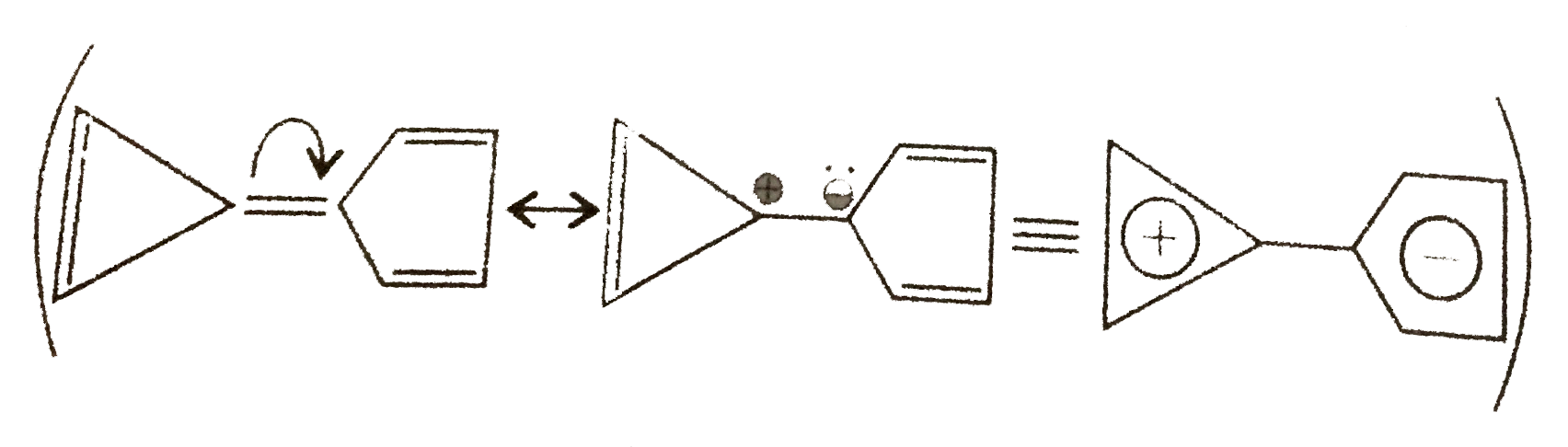
Same explanation as in 37.
39: Anti-aromatic: `8 vec(e)'s` system (`6pi vec(e)'s + 2vec(e)'s` from `LP` on `N`) in complete delocalisation, cyclic,planar adn follows `4n` rule `(n = 2)`.
40. Non-aromatic: `4vec(e)` system but not in compelete delocalisation, cylic palnar.
41. Aromatic:
`10pi vec(e)` system, in complete delocalisation, cycle plannar. In [10] annulene, interaction by the internal `H` atoms prevents it from achieving copanirty. Here in this compound. If atoms are replaced by methylene bridge `(-CH_(2)-)` above the ring, which makes it palnner.
Moreover, bridgehead `C` atoms are still `sp^(2)-` hybridised and theri `vec(e)'s` are available for extended delocalisation. Hence, aromatic.
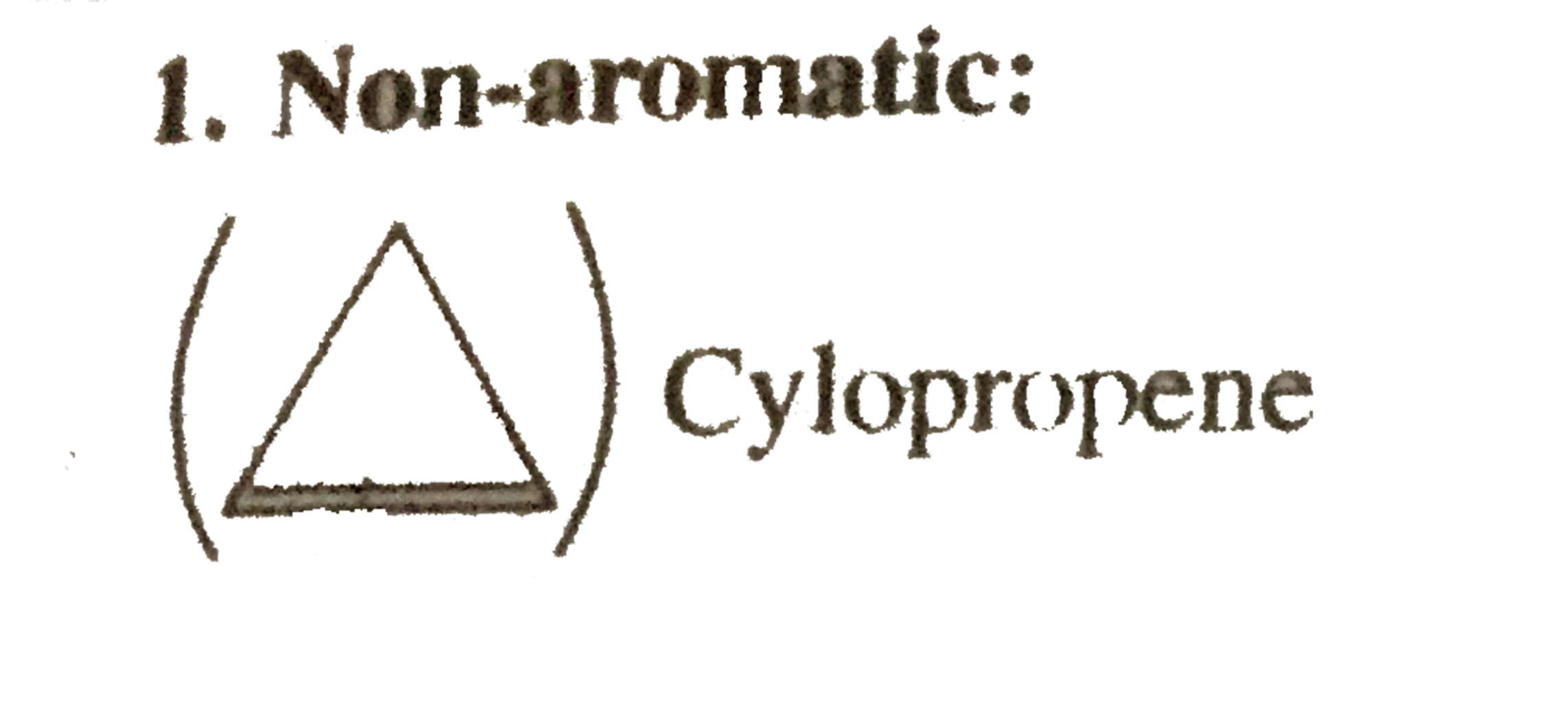 Cylopropene
Cylopropene Cyclic, planner, `2pi vec(e) s` system, folows `(4pi + 2)pi vec(e)` but not in delocalisation or not in resonance.
2. Aromic:
 Cylopropenyl cation
Cylopropenyl cation Cylic, planner `2pi vec(e)s` system follow `(4pi + 2) pi vec(e)s` and `2pi vec(e)s` are in delocalisation or in resonance with positive charge.
3. Anti-aromatic:
 Cycloprepenyl anion
Cycloprepenyl anion Cyclic, planner, `4 vec(e) s` system `(2pi vec(e) + 2 vec(e)` from one negative charge), follows `4n` rule and `2pi vec(e)s` are in conjugatioln with one negative charge and are in delocalisation or in resonance with negative charge.
4. Non-aromatic:
 Explained in Section 11.10 (c)`
Explained in Section 11.10 (c)` 5. Non-aromatic:
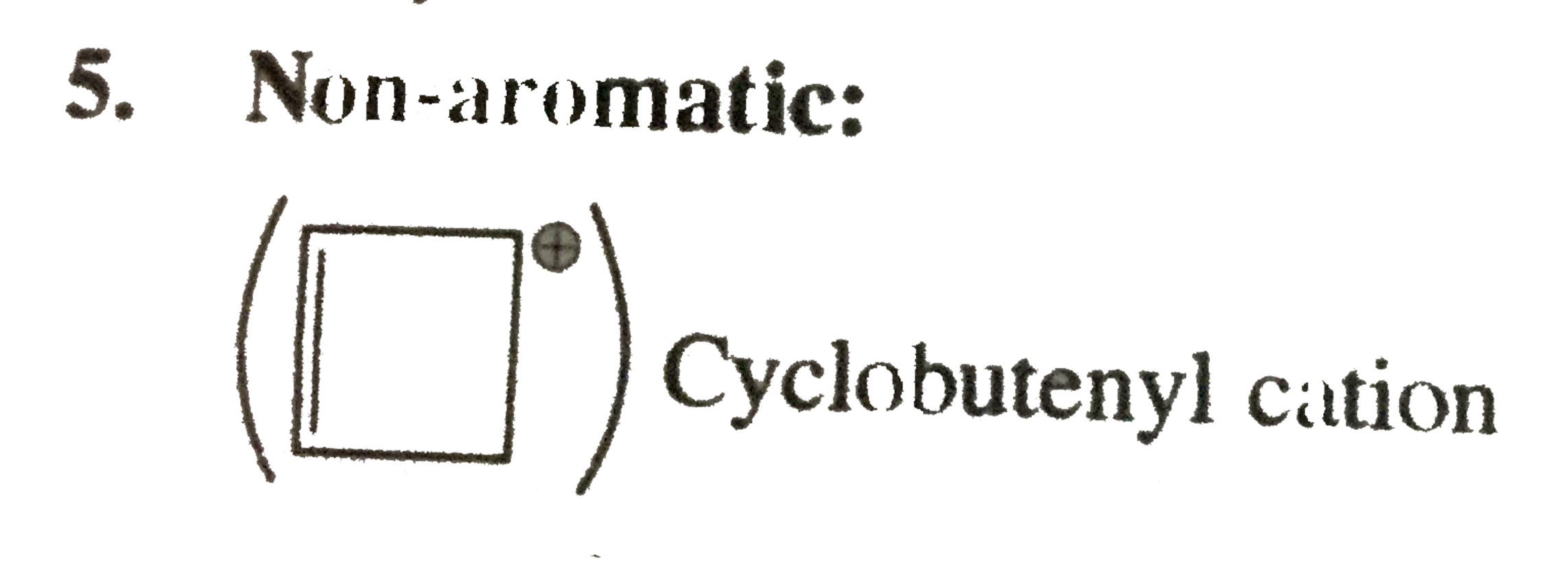 Cyclobutenyl cation
Cyclobutenyl cation Cyclic, plannar, `2pi vec(e)s` system, follows `(4n + 2) pi vec(e) s, (pi = 0)`, but `2pi vec(e)s` are not in complete delocation with positive charge.
6. Non-aromatic:
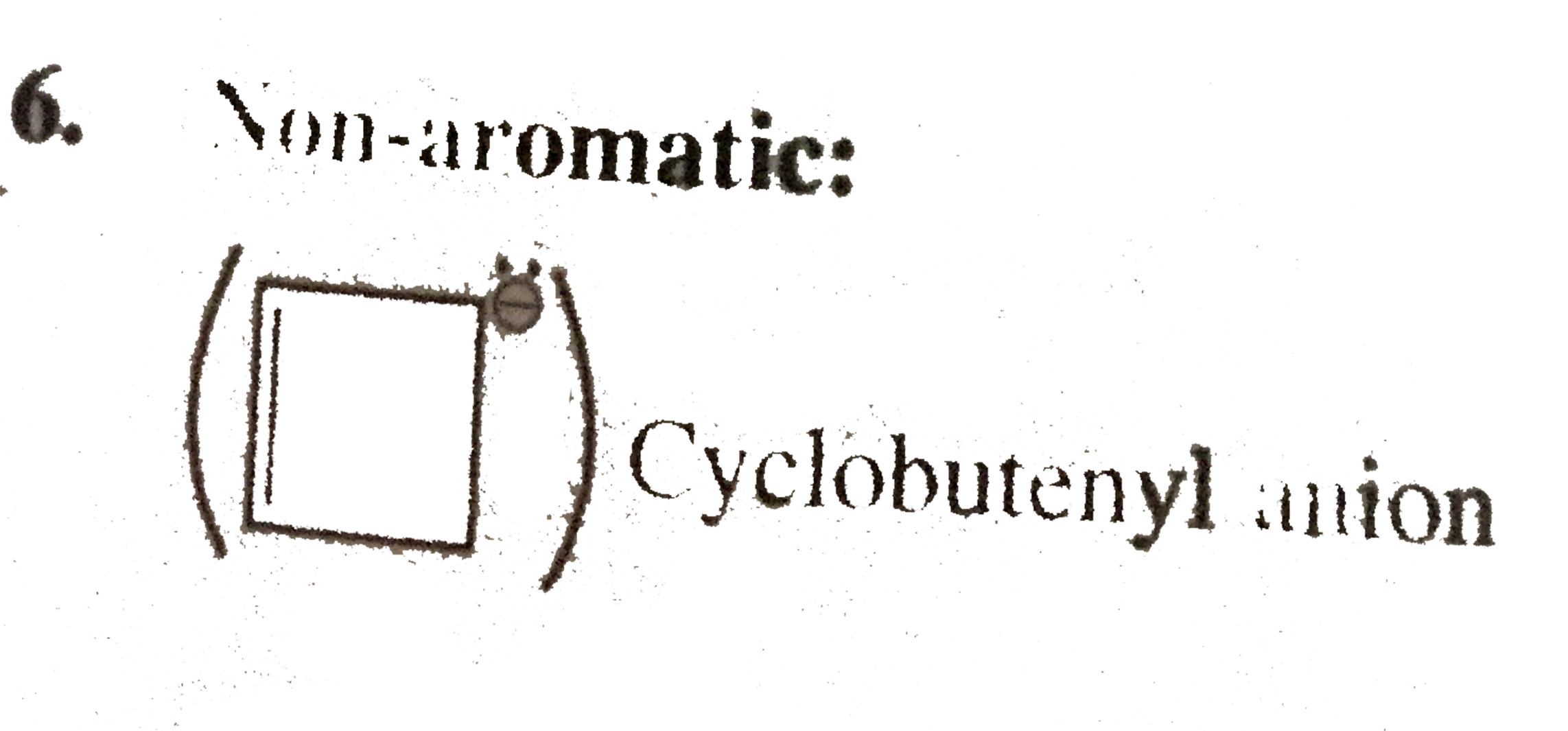 Cyclobutenyl anion
Cyclobutenyl anion Cyclic,n planar, `4pi vec(e)'s` system `(2pi vec(e) + 2 vec(e)` from one negative charge ) follows `4n` rule `(n = 1)` but `2pi vec(e)'s` are not in complete delocalisation with negative charge.
7. Aromatic:
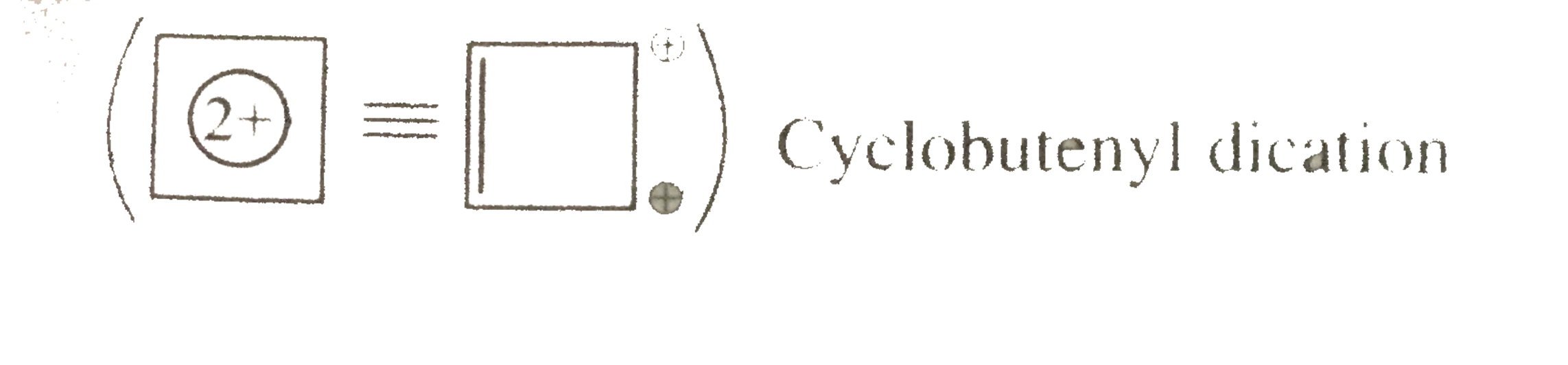 Cyclobutenyl direction
Cyclobutenyl direction Cylic, planar `@pibar(e)` 's system, following `(4n+2)` rule `(n=0)` , and `2pibar(e)` 's are in complete delocalisation or in resonance with two positive charges.
8. Aromaitc:
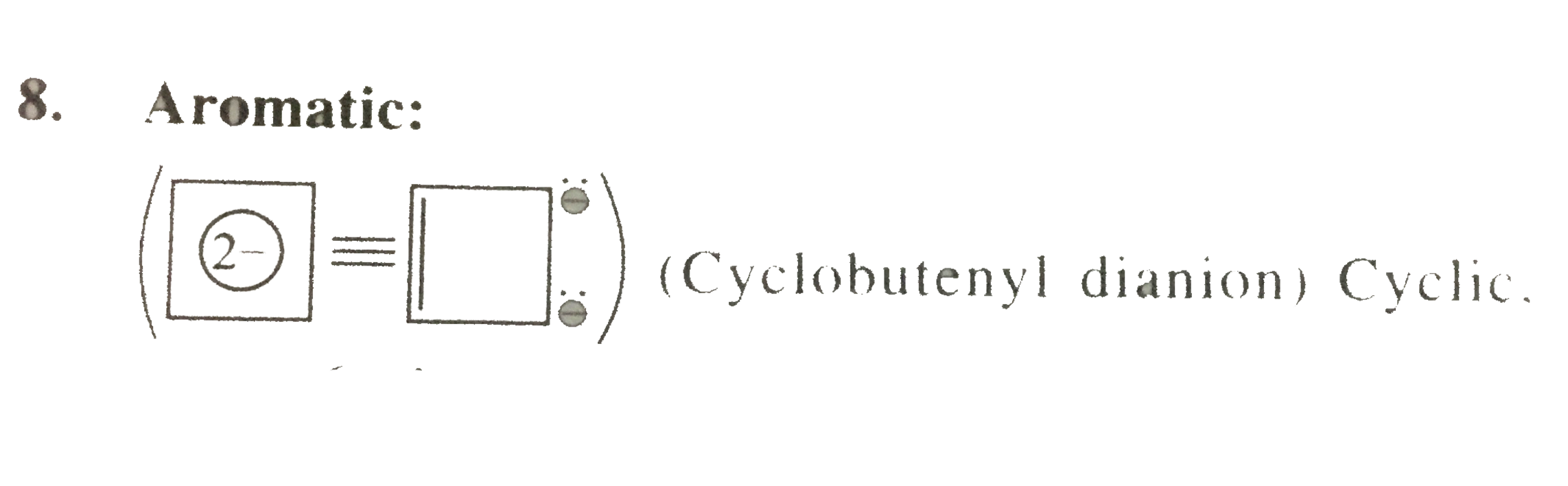 (Cyclobutenyl dianion) Cyclic, planar `6bar(e)` 's system `[2pibar(e)+4bar(e)` 's (from two negative charges) ] follows `(4n+2)` rule `(n=1)` ansd 2pibae(e)` 's are in negitive charges.
(Cyclobutenyl dianion) Cyclic, planar `6bar(e)` 's system `[2pibar(e)+4bar(e)` 's (from two negative charges) ] follows `(4n+2)` rule `(n=1)` ansd 2pibae(e)` 's are in negitive charges. 9. Non-aromatic:
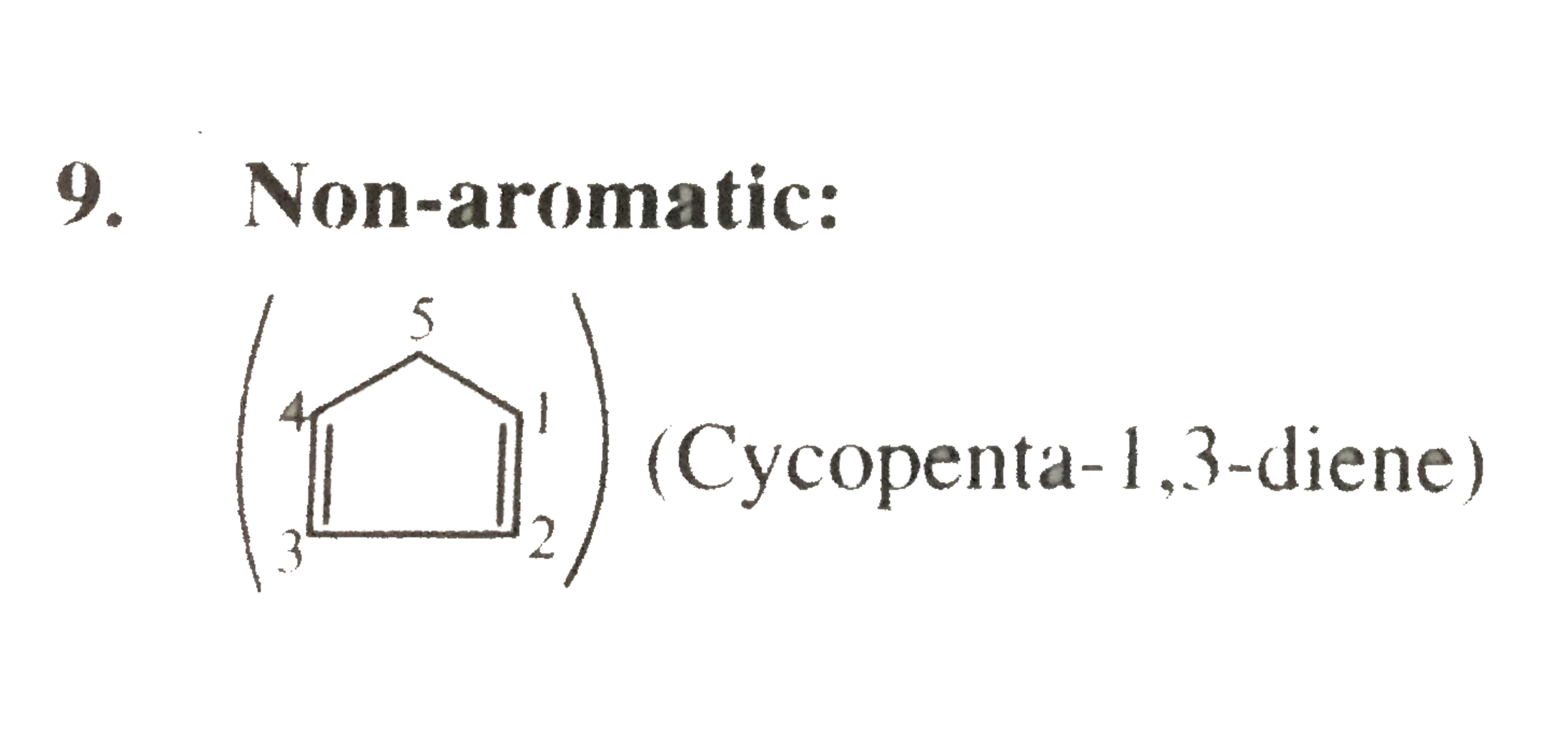 (Cycopenta `-1,3-` diene)
(Cycopenta `-1,3-` diene) Cyclic, planar `4pibar(e)` 's system, follows `4n `rule `(n=1)` ,. and `4pibar(e)` 's are mot in complete deloalisation or not in resonance.
10. Antiaromatic:
 (Cyclopenta `-1,3-` dienyl cation)
(Cyclopenta `-1,3-` dienyl cation) Cylic, planar, `4pibar(e)` 's system, follows `4n` rule `(n=1)` , and `4pibar(e)` 's are in complete delocalisation or in resonance with positive charge.
11. Aromatic:
 (Cyclopentea `-1,3-` dienyl anion)
(Cyclopentea `-1,3-` dienyl anion) Cycilc, planar `6bar(e)` 's system `(4pibar(e)` 's+2bar(e)` 's from one negative charge) follows `(4n+2)` rule `(n=1)` , and `4pibar(e)` 's are in complete deloclisation or in resonanace with negative charge.
12. Aromatic:
 (Benzene)
(Benzene) Cyclic, Planar, `6pibar(e)` 's system, follows `(4n+2)` rule `(n=1)` , `6pibar(e)` 's are in complete delocalisation or in resonace.
13. Non-aromatic:
 (Cyclohepta `-1,3,5-` triene)
(Cyclohepta `-1,3,5-` triene) Cyclic planar, `6pibar(e)` 's system, follows `(4n+2)` rule `(n=1)` , but `6pibar(e)` 's are not in complete delocalisation or not in resonance.
14. Aromatic:
 Cyclohepta `-1,3,5-` trienly cation
Cyclohepta `-1,3,5-` trienly cation Cyclic, planar `6pibar(e)` 's system, follows `(4n+2)` rule, `(n=1)` , and `6pibar(e)` are in complete delocalisation or in resonance with positve charge.
15.
 Cyclohepta `-1,3,5-` trienly anion
Cyclohepta `-1,3,5-` trienly anion Cyclic, planner `8vec(e) s` system (`6pi vec(e) + 2 vec(e)` from one negative charge) follows `4n` rule `(n = 2)`, and `6pi vec(e) s` are in complete delocalisation or in resonanance wtih negative charge.
16. Aromatic:

Cyclic, planner, `6pi, vec(e) s` of the ring, follows `(4pi + 2)` rule `(n = 1)` and `6pi vec(e)` are in complete delocalisation or in resonance with positive charge on the ring.
17. Aromatice:

Neither cyclophertarience nor cycloperntadience alone is aromatic but resonanace structure bears a positive charge in the secen-membered ring, given it an aromatic character of cycloherpratrientyl cation, while the five-membered ring bears a negative charge making it similar to the aromatic cyclopentadienty1 atom cyclopentadiency a anion. This cahrge sepration is reponsible for the observed dipole moment. Moreover, it is cyclic, planner with `10pi vec(e)s` are in complete decolaiation or in resonance.
18. Aromatic:

Cyclic planner, `6 vec(e)s` system (`4pi vec(e)'s + 2 vec(e)` from only one `I.P`) (only lone pair is required for delocalisation). Follows `(4pi + 2)` rule `(n = 2), 4pi vec(e)s` and `2 vec(e)'s` from `Lp` are in complete delocalistaion or in resonance hence aromatic.
19. Aromatic:
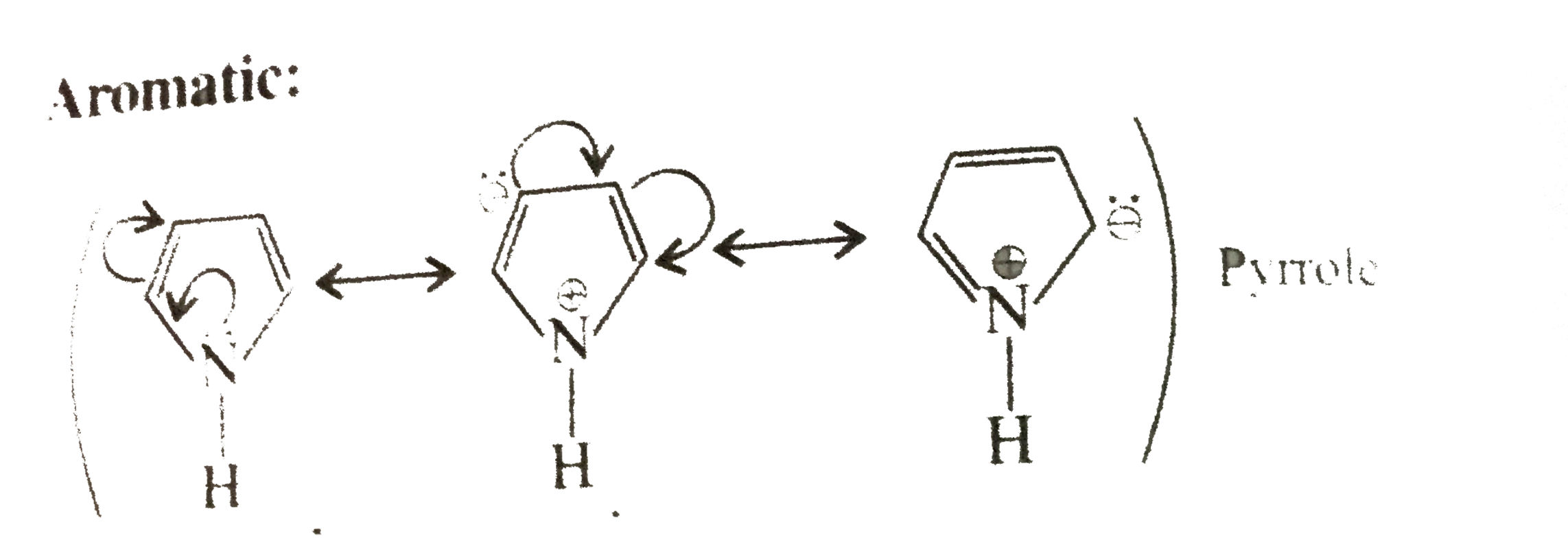
Same explanation as in18.
20. Aromatic:

Same explanation as in 18.
21. Aromatic:

(Lone pair `vec(e)'s` are not used in delocalisation)
Same explanation as in 18.
22. Aromatic:

Cylclic, planner, `10pi vec(e)'s` system, follows `(4n + 2)` rule `(n + 2)` in complete delocalisation or in resonance
23. Aromatic:

Cylic, planner, `14pi vec(e)'s` system follows `(4n + 2)` rule `(n = 3)`, in complete delocalisiton or resosnance.
24. Aromatic:

Same explanation as in 23. Cyclic palnner, `2pi vec(e)'s` of the ring in complete delocalisation or in resonance with positive charge on the ring, follows `(4n + 2)` rule `(n = 0)`.
26. Anti-aromatic `(overset(o+)(C)_(9) H_(9)):
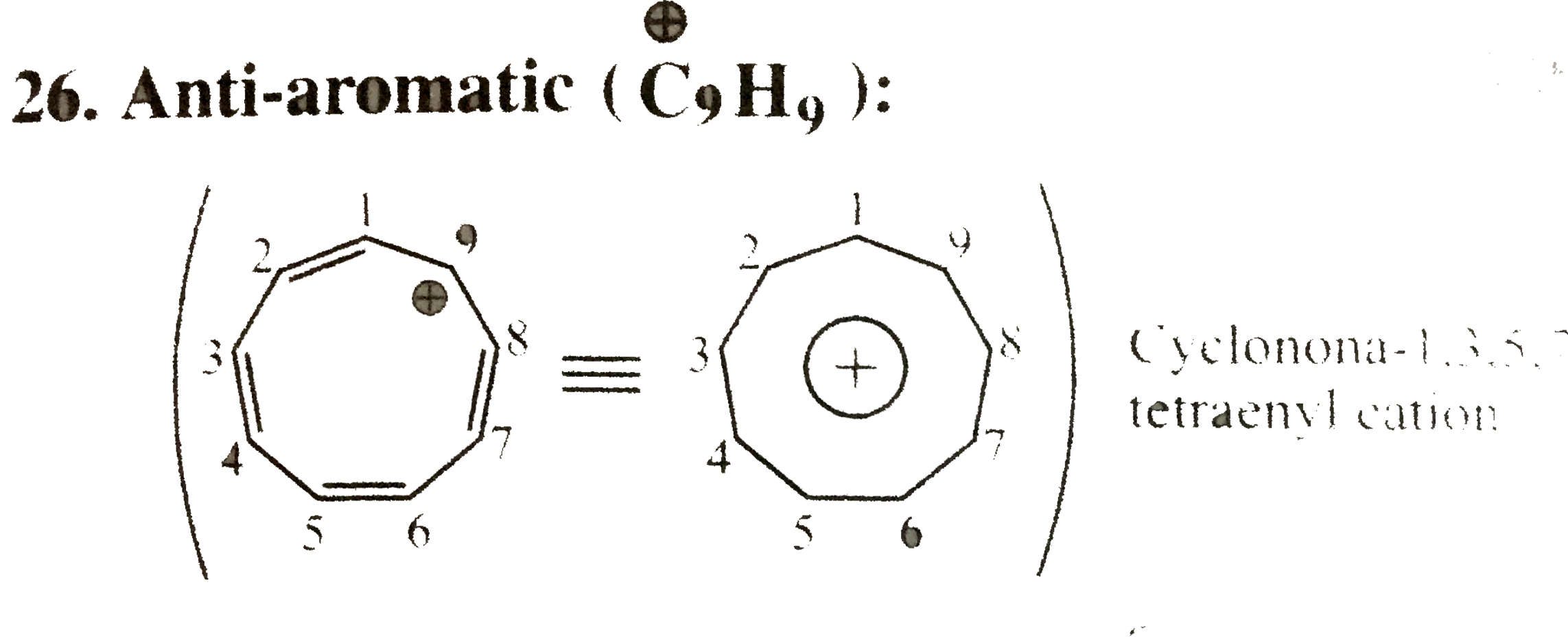
Cyclic, planar, `8pi vec(e)'s` system follows `4n` rule `(n = 2)` and `8pi vec(e)'s are in complete delocalisation or in resonance with positive charge.
27. Aromic`(overset(o-)(C)_(9) H_(9))`:

Cyclic planar, `10 vec(e)'s` system `[8pi vec(e)'s + 2 vec(e)` from one negativ charge)], follows `(4n + 2)` rule `(n = 2)`, and `8pi vec(e)'s` are in complate delocalisation or in resonance with negative charge.
28. Non-aromatic `(C_(9) H_(10))`:

Cyclic, planar, `8pi vec(e)'s` system, follows `4n` rule `(n = 2)`, but tehy are not in complete delocalisationor not in resonance.
29. Aromatic:

Cyclic, planner, both `N` and `B` are `sp^(2) - hybridised. Each `N` has two bonding `vec(e)'s (LP vec(e)'s)` in a `P`- orbital and each `B` has an emply `p`-orbital, giving a total of six' delocalises, `pi vec(e)'s`, hence aromatic, All `(B-N)` bond lenghths are equal an all ring angles are `120^(@)`.
30. Aromatic:

In isolated benene rings, `pi vec(e)'s` are counted seperately in each ring unlike the fused rings, in whcih total `pi vec(e)'s` are counted to verify `(4n + 2)` or `4n` rule.
In biphenyl, each ring has six `pi vec(e)'s` in delocalsation, hence aromatic.
31. Aromatic:

Ring (I) has six `pi vec(e)'s` in delocalisation and is aromatic. But rign (II) has only two `pi vec(e)'s` but not in delocalisation, so ring (II) is non-aromatic. In isolated ring. If one ring is aromatic the compound is said to be aromatic.
32. Aromatic:
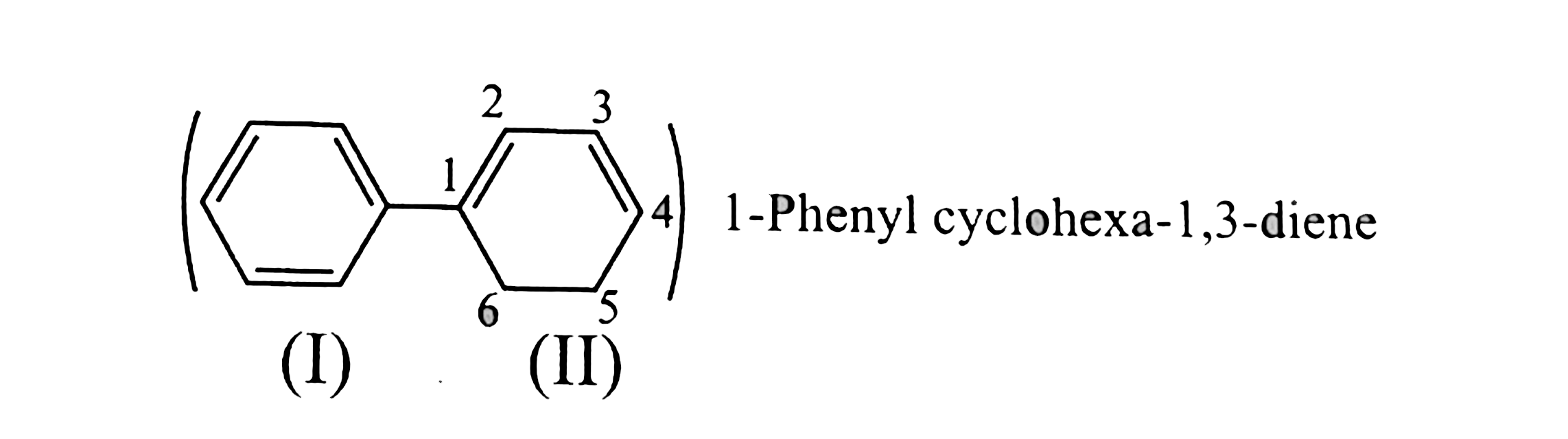
Ring (1) has six `pi vec(e)'s` in deloclisationand is aromatic. But ring (ii) has only four `pi vec(e)'s` but not is delocalisation and is non-armatic. But at least one ring is aromatic, so the compound is armotic.
33. Aromatic:

same explanation as in `30-32`.
34. Non-armatic:

It is not planer but tub-shaped. The `p`-orbits of one `C = C)` are not coplaner with those of a negihbouring `(C = C)` and there can be no effective overlap for delocalisation. The `8pi vec(e)'s` system follows `4n` rule `(n = 2)`, but due to non-planar, it is not anti-aromonic.
35. Anti-aromatic:

(II) is `4pi vec(e)'s` system in conjugation with complete delocalisation, follows `4n` rule `(n = 1)`, adn is anti-aromatic.
36. Aromatic:
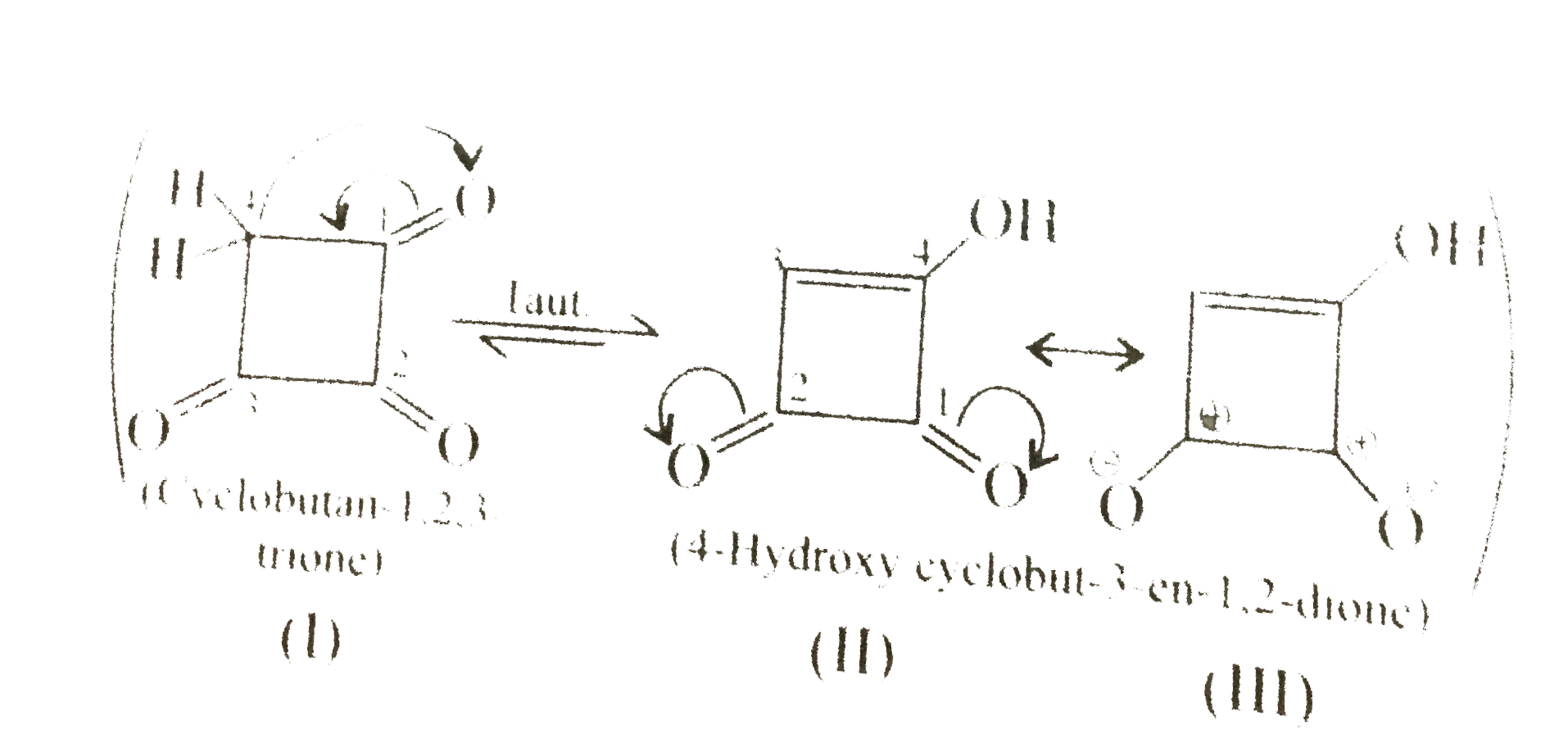
(III) is cyclic, plannar, `2pi vec(e)'s` system and in complete delocalisation wtih two positive charges on the ring (similar to cyclobutenyl dication). It follows `(4n + 2)` rule `(n = 0)`.
37. Aromactic:

Neither the five-membered not the seven-membered ring alone is aromatic character of cycloheptatrieny' cation, while the five-membered ring bears a negative charge making it similar to the aromatic cyclopentadienyl anion. This charge separation is reponsible for the observed dipole moment. Further, each ring is aromatic.
38. Aromatic:

Same explanation as in 37.
39: Anti-aromatic: `8 vec(e)'s` system (`6pi vec(e)'s + 2vec(e)'s` from `LP` on `N`) in complete delocalisation, cyclic,planar adn follows `4n` rule `(n = 2)`.
40. Non-aromatic: `4vec(e)` system but not in compelete delocalisation, cylic palnar.
41. Aromatic:
`10pi vec(e)` system, in complete delocalisation, cycle plannar. In [10] annulene, interaction by the internal `H` atoms prevents it from achieving copanirty. Here in this compound. If atoms are replaced by methylene bridge `(-CH_(2)-)` above the ring, which makes it palnner.
Moreover, bridgehead `C` atoms are still `sp^(2)-` hybridised and theri `vec(e)'s` are available for extended delocalisation. Hence, aromatic.
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|21 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Problems|37 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 Videos
Similar Questions
Explore conceptually related problems
Non-aromatic compound is
Calssify the following as aromatic, antiaromatic and nonaromatic compounds.
Naphthalene is an aromatic compound.
Which one is not aromatic compound ?
The aromatic compound would be
Mention the important characteristics of aromatic compounds.
The aromatic compound would be :
What are the conditions for aromatic compounds.
Which of the following is a non-aromatic compound ?
CENGAGE CHEMISTRY ENGLISH-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Archives Subjective Type
- Select the aromatic, anti-aromatic, and non-aromatic a compounds. 1....
Text Solution
|
- Write the structure of the major organic product expected form the f...
Text Solution
|
- Complete the following giving sturctures of the principle organic pr...
Text Solution
|
- Give reasons for the following:
Text Solution
|
- What woule be the major product in each of the following reactions?
Text Solution
|