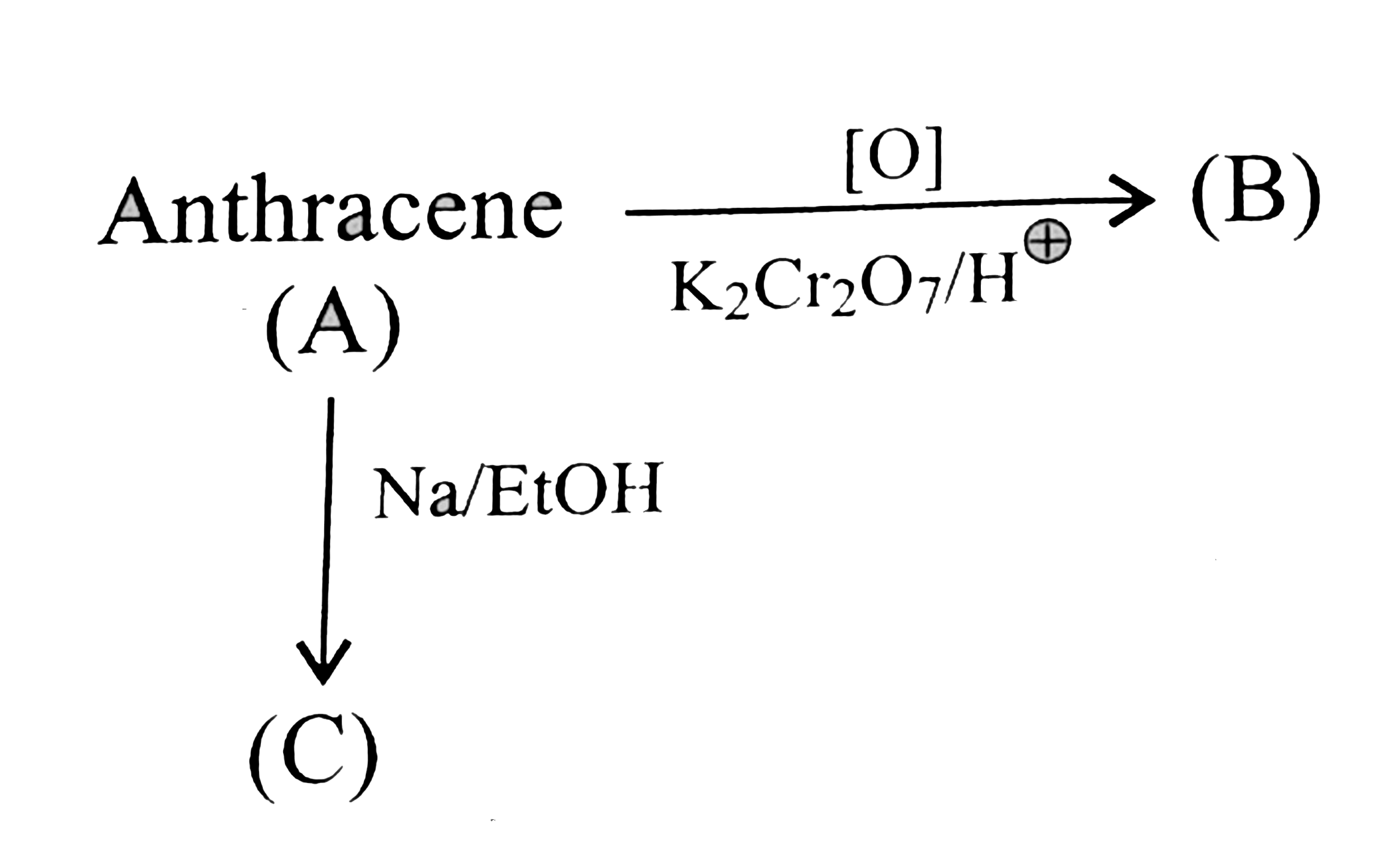
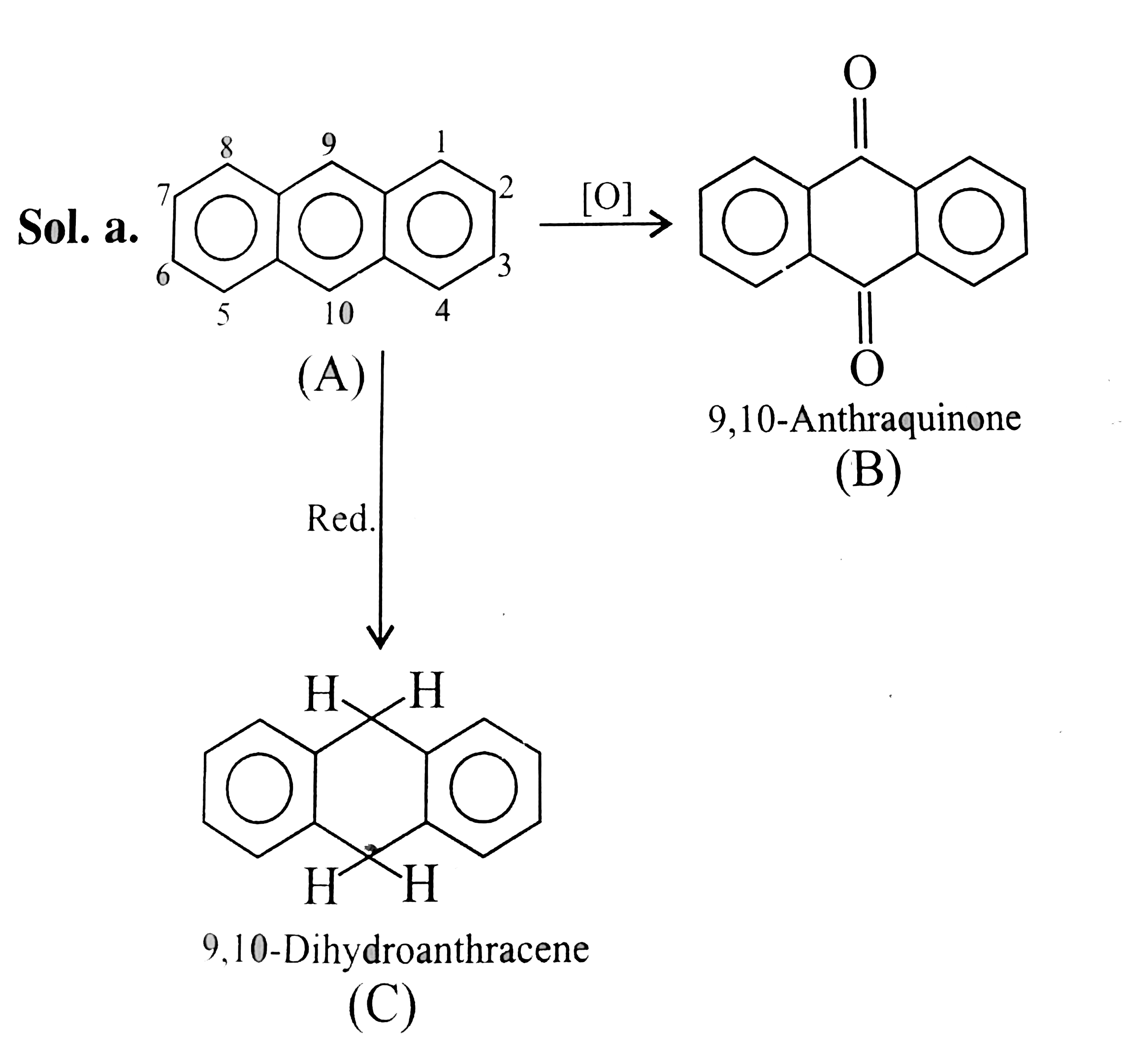
Text Solution
Verified by Experts
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|21 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Problems|37 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Archives Subjective Type
- a. Compare the following reaction.
Text Solution
|
- Write the structure of the major organic product expected form the f...
Text Solution
|
- Complete the following giving sturctures of the principle organic pr...
Text Solution
|
- Give reasons for the following:
Text Solution
|
- What woule be the major product in each of the following reactions?
Text Solution
|