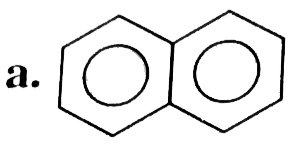
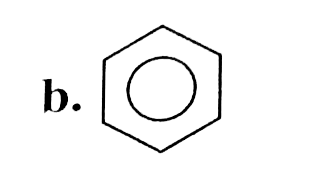
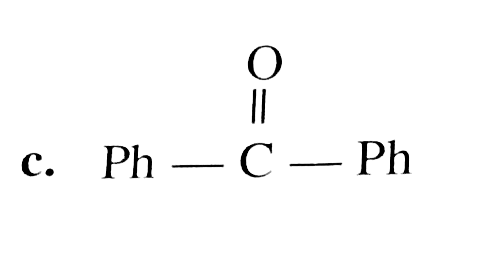
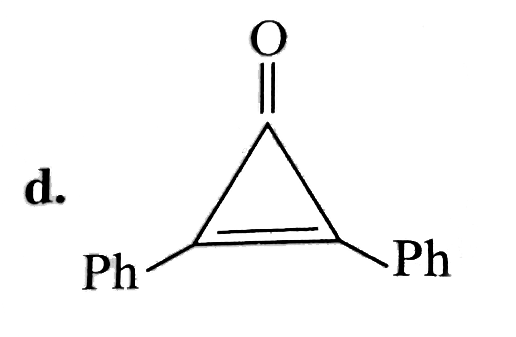
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Resoning Type|1 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Resoning|11 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct Answer Type|28 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Exercises Single Correct
- In the follwing reactionsL the rate of reaction (I) and (II) are...
Text Solution
|
- In which case will SE not be in m- position?
Text Solution
|
- Which of the folliwng has the highest dipole moment?
Text Solution
|
- Which of the following is the most stable carbonium or benzenium ion?
Text Solution
|
- Which of the following ketonic compound is the least stable?
Text Solution
|
- Which of the following compounds will give curdy precipate with AgNO(3...
Text Solution
|
- Which of the folliwng compounds is the most reactive towards electroph...
Text Solution
|
- Which of the folliwng is the most stable species?
Text Solution
|
- Which of the following in not aromatic in nature?
Text Solution
|
- Which of the following species will be least stable?
Text Solution
|
- Which of the follwing compounds will undergo Friedel Creadfts alylatio...
Text Solution
|
- Which of the following is anti-aromatic in nature?
Text Solution
|
- Which of the following aromatic compounds is least reactive towards el...
Text Solution
|
- Which of the folliwng compounds will show faster ArSN^(2) reactions?
Text Solution
|
- Which of the folliwng is the order of the rate of reaction of C(6)H(6)...
Text Solution
|
- Which of the folliwng is the correct order of the order of the rate of...
Text Solution
|
- Which of the following will give SN^(2) mechanism?
Text Solution
|
- Which of the following compounds gives SN^(1), SN^(2) and SN^(2) mecha...
Text Solution
|
- Which of the folliwning ether wil always give SN^(2) mechanism in acid...
Text Solution
|
- Which of the follwoign ether will always give SN^(2) mechanism in acid...
Text Solution
|