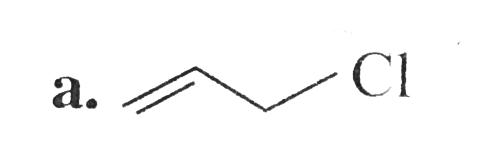
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Resoning Type|1 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Resoning|11 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct Answer Type|28 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Exercises Single Correct
- Which is the most effective ion in an SN^(2) displacement on methyl br...
Text Solution
|
- Of the following four groups are m- directing when present on a benzen...
Text Solution
|
- The chemistry of benzene is characterised by which fo the following t...
Text Solution
|
- A reaction involving an aromatic nucleas is usually initiated by:
Text Solution
|
- Which of the following deactivates the aromatic nucleas?
Text Solution
|
- The experimental determined rate equiaction for the alkaline hydrolys...
Text Solution
|
- Which of the following undergoes nitration most easily?
Text Solution
|
- Which of the follwing reaction s is not sterospecific?
Text Solution
|
- Which of the following reagents on reaction with acetylene yield same ...
Text Solution
|
- Which is least reactive towards nucleophilic substitution (S(N^(2)))
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory
Text Solution
|
- CH(3)Br +Nu^(Theta) rarr CH(3)-Nu +Br^(Theta) The decreasing order o...
Text Solution
|
- HBr reacts with overset(CH(2))overset(||)CH-OCH(3) at room temperature...
Text Solution
|
- The halogen compound which most readily undergoes nucleophilic substit...
Text Solution
|
- The correct decreasing order of SN^(1) reactivity of the folliwng is:...
Text Solution
|
- Which of the follwing has the highest boiling point?
Text Solution
|
- Which of the following sequence of reactions (reagents) can be used fo...
Text Solution
|
- The decreasing order of dipole moment of the folliwng is: I. CH(3)Cl...
Text Solution
|
- Bottles containing C6H5I and C6H5CH2I lost their original labels. They...
Text Solution
|
- One mole of 1,2-dibromopropane on treatment with X moles off NaNH(2) f...
Text Solution
|