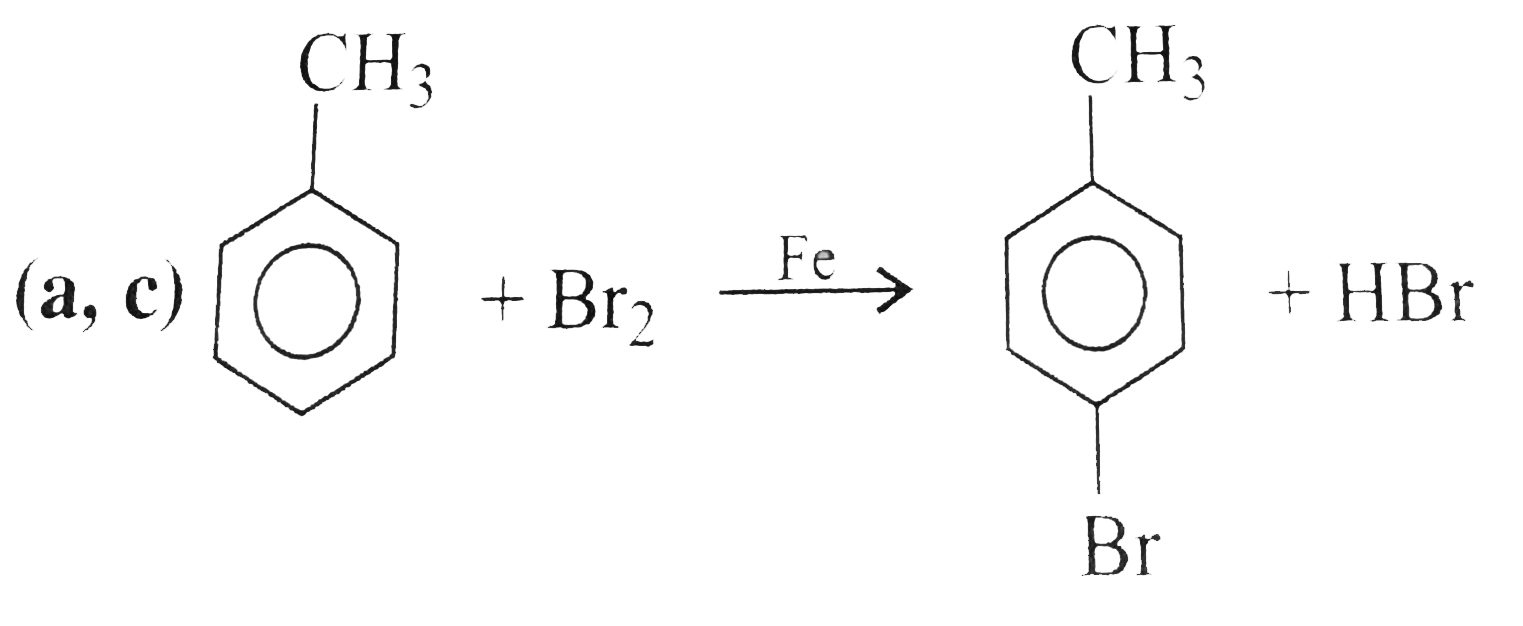A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Mutiple Correct Answers Type|1 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In Theblanks|12 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Single Correct Answer Type|3 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Archives Mutiple Correct
- An aromatic molecule will
Text Solution
|
- Toluene, when treated with Br(2)Fe, gives o and p-bromotoluene, becaus...
Text Solution
|
- Aryl halides are less reactive towards nucleophilic substitution react...
Text Solution
|
- The compounds used as refrant are:
Text Solution
|
- The products of reaction of alcoholic silver nitrate with ethyl bromid...
Text Solution
|