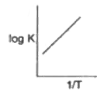
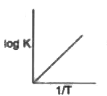
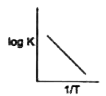
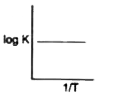
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RC MUKHERJEE-CHEMICAL EQUILIBRIUM-Objective Problems
- Which of the curves given in Q. 36 represents a standard reaction for ...
Text Solution
|
- Which of the curves given in Q. 36 represents a standard reaction with...
Text Solution
|
- Each of the mixtures listed below was placed in a closed container and...
Text Solution
|
- Consider the following equilibrium in a closed container N(2)O(4)(g)...
Text Solution
|
- The value of the reaction quotient before any reaction occurs is
Text Solution
|
- What is the minimum mass of CaCO3(s), below which it decomposes comple...
Text Solution
|
- The energy profile of the reaction: is shown as A+B Leftrightarrow C i...
Text Solution
|
- NiO is to be reduced to Ni in an industrial process by the use of the ...
Text Solution
|
- Which of the following curves between log K and 1/T is correct?
Text Solution
|
- The thermal dissociation of equilibrium of CaCo(3)(s) is studied under...
Text Solution
|
- For the reaction, SO(2)(g) + (1)/(2)O(2)(g)hArrSO(3) (g), If K(p) = K(...
Text Solution
|
- The following reaction is performed at 298 K ? 2NO(g)+O(2)(g)hArr2NO...
Text Solution
|
- The standard Gibbs energy change at 300K for the reaction 2AhArrB+C is...
Text Solution
|
- The percentage yield of ammonia is a function of time in the reaction ...
Text Solution
|
- The equilibrium constant at 298K for a reaction, A+BhArrC+D is 100. If...
Text Solution
|
- Thermal decomposition of gaseous X, to X at 298 K takes place accordin...
Text Solution
|
- The incorrect statement among the following for this reaction is
Text Solution
|
- Which of the following lines correctly show the temperatrue dependence...
Text Solution
|
- At a certain temperture in a 5L vessel 2 moles of carbon monoxide and ...
Text Solution
|
- In which of the following reactions, an increase in the volume of the ...
Text Solution
|