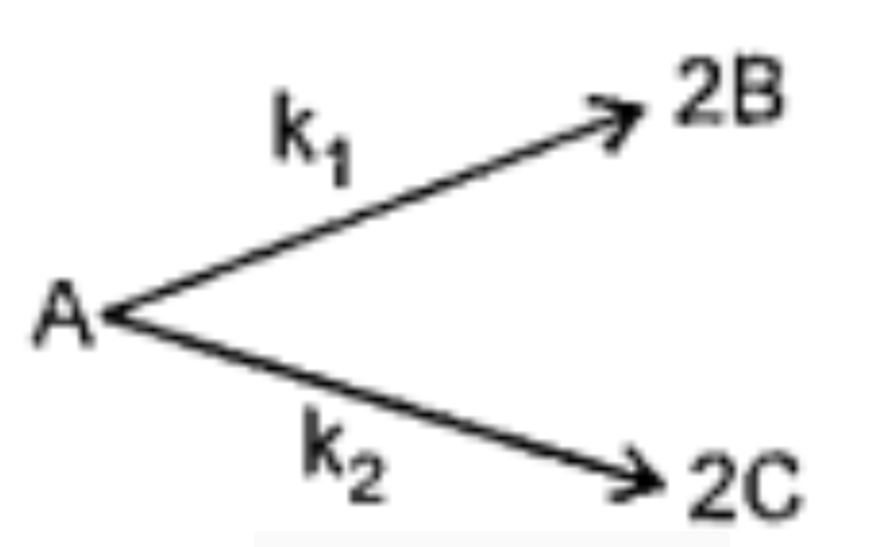
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- The rate of the chemical reaction doubles for an increase of 10K in ab...
Text Solution
|
- The activation energy for the reaction, 2 HI (g) to +I-2(g), is 209.5 ...
Text Solution
|
- For the elementary reaction where k1/k2 = 1/2 Initially only 4 mol...
Text Solution
|
- For the sequently reaction A overset (KA) to B overset (KB) to C rat...
Text Solution
|
- If the atomic masses of lithium, helium and proton are 7.01823 amu, 4....
Text Solution
|
- Calculate the mass defect and binding energy per nucleon for "(27) Co^...
Text Solution
|
- Calculate the energy evolved (in joutes) per molecule of helium by the...
Text Solution
|
- Which of the following elements is non radioactive ?
Text Solution
|
- Predict the most likely mode of decay and product of decay for the nuc...
Text Solution
|
- Find out the total number of alpha and beta particles emitted in the d...
Text Solution
|
- Ten gram atoms of an alpha-active radioisotope are disintegrating in a...
Text Solution
|
- The half-life of Th^(232) is 1.4xx10^(10) years and that of its daught...
Text Solution
|
- Which of the following nuclide is likely to be radioactive?
Text Solution
|
- A piece of wood was found to have C^(14)/C^(12) ratio 0.7 times that i...
Text Solution
|
- In a sample of uranium ore the weight of Pb^(206) is 20.6% of the weig...
Text Solution
|
- The rate of the elementary reaction 2NO +O2 to 2NO2 when the volume of...
Text Solution
|
- For a general chemical change 2A =3B to products, the rates with respe...
Text Solution
|
- For the reaction N2 O5 to 2NO2 + 1/2 O2. Given -(d[N2O5])/dt = k3 [N2...
Text Solution
|
- Which of the following statement is false
Text Solution
|
- if a is the initial concentration then time required to decompose hafe...
Text Solution
|