A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- Which of the following statement is false
Text Solution
|
- if a is the initial concentration then time required to decompose hafe...
Text Solution
|
- What will be the order of reaction for a chemical change having follow...
Text Solution
|
- The rate constant for the reaction, 2N2O5to 4NO2+3.0xx 10^-5s^-1.if th...
Text Solution
|
- Which is correct relation between dc/dt,dn/dt and dp/dt, where c,n,p r...
Text Solution
|
- For a first order reaction the ratio of times to complete 99.9% and ha...
Text Solution
|
- The decomposition of a hydrocarbon follows the equation k= (4.5 xx 10^...
Text Solution
|
- A reaction (A) forms two products Ea2 =2Ea1 than K1 and K2 will be...
Text Solution
|
- The activation energy of a reaction is 12.89 kcal/mol. The increase in...
Text Solution
|
- A to B DeltaH=-10KJ mol, Ea=50KJ // mol then activation energy of B t...
Text Solution
|
- The rate constant, the activation energy and the arrameter parameter o...
Text Solution
|
- For an exothermic chemical process occurring in two steps as: (i)A+B r...
Text Solution
|
- if activation energy of a reaction is zero
Text Solution
|
- For an exothermic reaction where Delta H represents the enthalpy of th...
Text Solution
|
- The decomposition of phosphine (PH3) an tungsten at low pressure is a ...
Text Solution
|
- The rate of a first order reaction is 0 04 mol L^-1s^-1 at 10 seconds ...
Text Solution
|
- The addition of a catalyst during a chemical reaction alters which of ...
Text Solution
|
- Which of the statements (1) - (4) about the reaction profile below is ...
Text Solution
|
- 75% of a first order reaction was completed in 32 min. When would 50% ...
Text Solution
|
- Higher order (gt3) reactions are rare due to
Text Solution
|
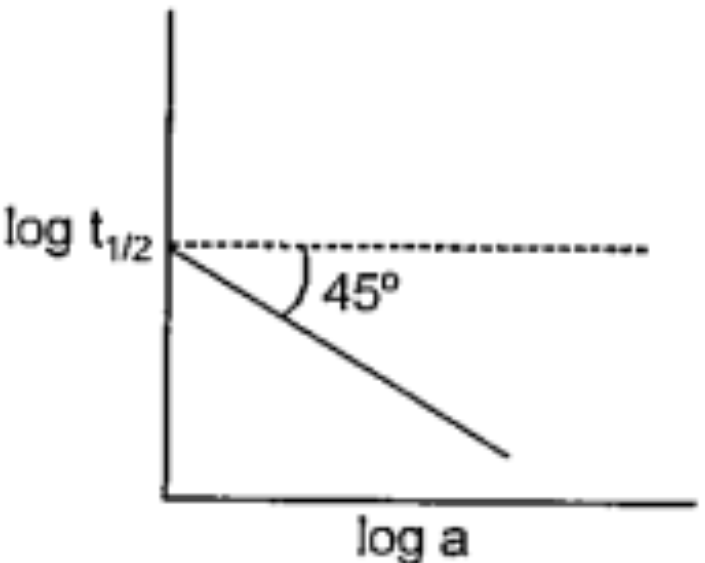 (a = initial concentration of reaction: `t_1/2`= half-life)
(a = initial concentration of reaction: `t_1/2`= half-life)