A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- For the non - stoichometric reaction 2A +B to C+D, the following kinet...
Text Solution
|
- The rate of a certain reaction is given by, rate = k [H^+]^n. The rate...
Text Solution
|
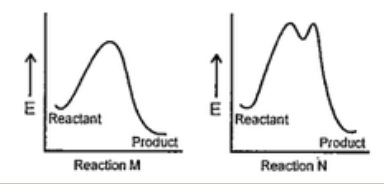
- The correct statement regarding the following energy diagrams is
Text Solution
|
- A piece of wood from an archaeological sample has 5.0 counts min^-1per...
Text Solution
|
- During the emission of a positron from a nucleus, the mass number of t...
Text Solution
|
- beta emission is always accompanied by
Text Solution
|
- ""(98)Cf^(246) was formed along with a neutron when an unknown radioac...
Text Solution
|
- An atomic nucleus having low n/p ratio tries to find stability by
Text Solution
|
- A plot of 1/T vs Ink for a reaction gives the slope -1xx10^4K. The ene...
Text Solution
|
- A(g) overset (triangle) to P(q)+Q(g)+R(g), follows first order kinetic...
Text Solution
|
- The half life period for a first order reaction is .......
Text Solution
|
- According to Arrhenius equation, the slope of log k vs 1/T plot is
Text Solution
|
- The value of rate constant for a first order reaction is 2.303 xx 10^-...
Text Solution
|
- Rate law for the reaction A + B to product is rate= k[A]^2{B]. What is...
Text Solution
|
- The rate of a reaction doubles when its temperature changes from 300 K...
Text Solution
|
- The rate of reaction is doubled for every 10^0 rise in temperature. Th...
Text Solution
|
- The conversion of A to B follows second order kinetics, Doubling the c...
Text Solution
|
- 75% of a first order reaction is completed in 30 min. What ts the time...
Text Solution
|
- Order of reaction is decided by
Text Solution
|
- A chemical reaction was carried out at 300 K and 280 K. The rate const...
Text Solution
|