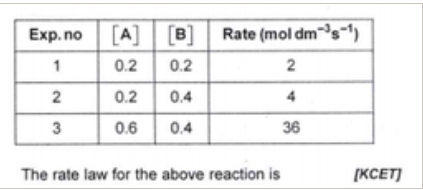
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- The rate of reaction is doubled for every 10^0 rise in temperature. Th...
Text Solution
|
- Activation energy (Ea) and rate constants (k1 and k2) of a chemical re...
Text Solution
|
- For a reaction, A+ B to products, the rate of the reaction at various ...
Text Solution
|
- For the first order reaction, rate constant depends upon
Text Solution
|
- Units of specific reaction rate for second order reaction is
Text Solution
|
- Half-life of a reaction is found to be inversely proportional to the c...
Text Solution
|
- Assertion : The order of reaction may be defined as the sum of the pow...
Text Solution
|
- Assertion : Acid catalysed hydrolysis of ethyl acetate is a first orde...
Text Solution
|
- For which order half life period is independent of initial concentrati...
Text Solution
|
- For a reaction 1/2Ararr2B, rate of disappearance of 'A' is related to ...
Text Solution
|
- For a chemical reaction, aA=bBrarrcC=dD. The ratio of rate of disappea...
Text Solution
|
- A drop of solution (volume=0.05 ml) contains 6 x 10^-7 mol of H^+. If ...
Text Solution
|
- In a reversible chemical reaction, 2NO2 overset(k1) underset(k2) iff N...
Text Solution
|
- In a second order reaction, first order in each reactant A and B, whic...
Text Solution
|
- The rate constant for the reaction 2N2O5rarr4NO2+O2 is 3xx10^-5 sec^-1...
Text Solution
|
- Consider a gaseous reaction, the rate of which is given by k[A][B], th...
Text Solution
|
- The relation between k1,k2 and k3 may be given as:
Text Solution
|
- For a reversible reaction of type mA iff nB, it was found that the con...
Text Solution
|
- What is the unit of rate constant of n^(th) order reaction?
Text Solution
|
- For a reaction A+BrarrC consider the following data:
Text Solution
|