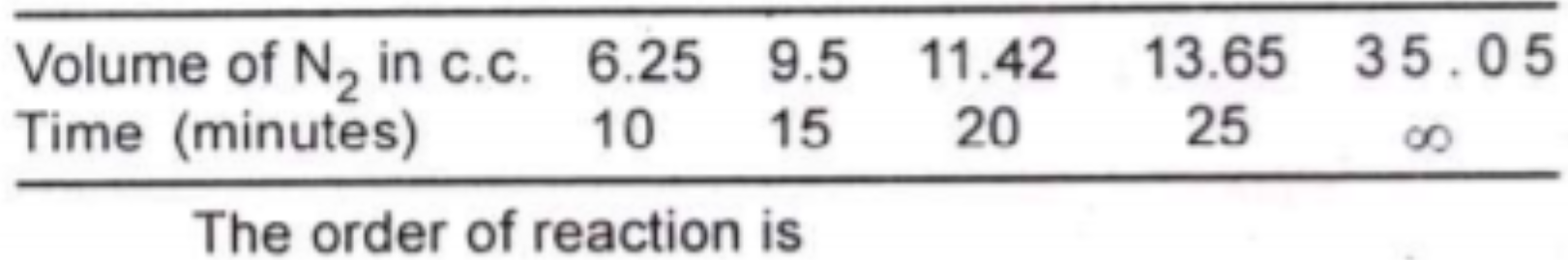
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- Half of a substance is consumed in 40 minutes. When the quantity of th...
Text Solution
|
- For a first order reaction, consider the graph below.
Text Solution
|
- The following data are for the decomposition of ammonium nitrite in aq...
Text Solution
|
- The half life period of a first order chemical reaction is 6.93 minute...
Text Solution
|
- The inversion of cane sugar proceeds with half-life of 500 minutes at ...
Text Solution
|
- Two first order reactions proceed at 25^@C, at the same rate. The temp...
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as : k=(Ae)^...
Text Solution
|
- The temperature coefficient for the saponification of ethyl acetate by...
Text Solution
|
- A catalyst lowers the activation energy of a reaction from 20 kJ/mole ...
Text Solution
|
- The activation energy for the reaction 2HIrarrH2+I2 is 184 kJ/ mole. H...
Text Solution
|
- The energies of activation for forward and reverse reaction for A2 + B...
Text Solution
|
- Consider an endothermic reaction Xrarr Y with the activation energies ...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- If a reaction A+BrarrC is exothermic to the extent of 30 kJ/mol and th...
Text Solution
|
- In a reaction, A+ B to product, rate is doubled when the concentration...
Text Solution
|
- The rate of change of concentration of (A) for reaction: ArarrB is gi...
Text Solution
|
- The following graph shows low t(1/2) (half-life) of a reactant R chang...
Text Solution
|
- Consider the following first order reaction: The percentage of 'B' i...
Text Solution
|
- Complete the following reaction
Text Solution
|
- The reaction, N2O5"(in" CCl4)rarr 2NO2+1/2O2(g) is first order in N2O5...
Text Solution
|