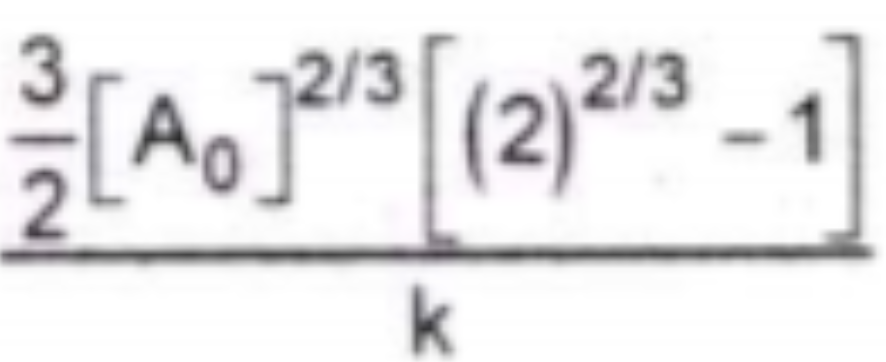
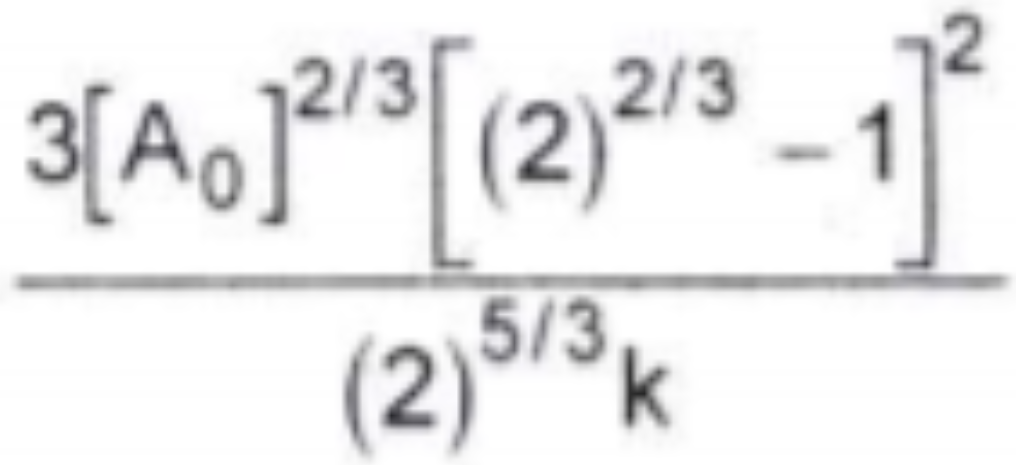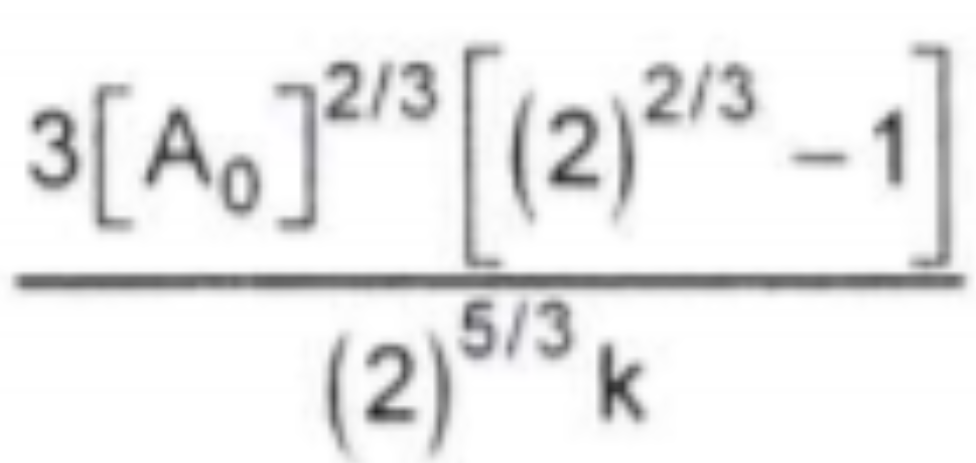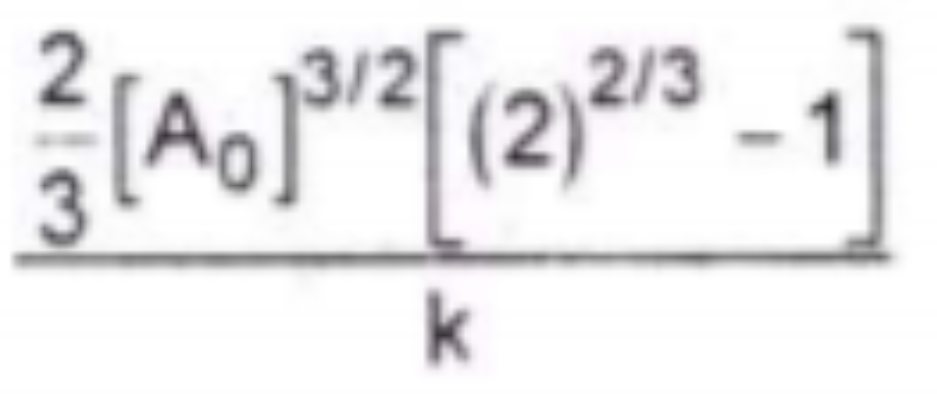A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- If a reaction A+BrarrC is exothermic to the extent of 30 kJ/mol and th...
Text Solution
|
- In a reaction, A+ B to product, rate is doubled when the concentration...
Text Solution
|
- The rate of change of concentration of (A) for reaction: ArarrB is gi...
Text Solution
|
- The following graph shows low t(1/2) (half-life) of a reactant R chang...
Text Solution
|
- Consider the following first order reaction: The percentage of 'B' i...
Text Solution
|
- Complete the following reaction
Text Solution
|
- The reaction, N2O5"(in" CCl4)rarr 2NO2+1/2O2(g) is first order in N2O5...
Text Solution
|
- For xA+yB rArr zC, - (d[A])/(dt) = (d[B])/(dt) = 1.5 (d[C])/(dt) then ...
Text Solution
|
- A reaction follows the given concentration-time graph The rate for thi...
Text Solution
|
- The decomposition of NO2 at 400K proceeds at a rate of 5.4 xx 10^-5 mo...
Text Solution
|
- Convert Aniline to m-Bromonitrobenzene.
Text Solution
|
- Which of these factors will affect the specific reaction rate of the g...
Text Solution
|
- Consider that the first order decomposition reaction of N2O5 written a...
Text Solution
|
- Which of the following statements is incorrect
Text Solution
|
- The rate law for a reaction between the substances A and B is given by...
Text Solution
|
- The following data are for the reaction, A+Brarr products
Text Solution
|
- For the reaction: H2(g) + Br2(g) rarr 2HBr(g) the experimental data su...
Text Solution
|
- A hypothetical reaction A2 + B2 rarr 2AB follows the following given m...
Text Solution
|
- The unit of rate of reaction and rate constant are identical for a
Text Solution
|
- For a zero order reaction, the plot of [A]t versus t for the reaction ...
Text Solution
|