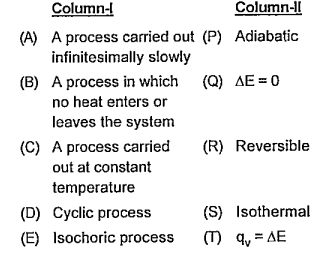
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
PATHFINDER-CHEMICAL KINETICS-QUESTION BANK
- In the Arrhenius equation, k =Ae^(Ea/RT), the rate constant (k) become...
Text Solution
|
- Amides may be converted into amines by a reaction named after::
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- Why addition of electrolyte is commonly used for destruction of colloi...
Text Solution
|
- Match the Column-I with Column-II
Text Solution
|
- The half-life of decomposition of PH, for different initial pressures ...
Text Solution
|
- A reaction X2(g) to Z(g) + 1/2 Y(g) exhibits an increase in pressure f...
Text Solution
|
- Aqueous solution of ammonia consists of:
Text Solution
|
- If the rate of reaction is 2.6xx10^-3 mol L^-1s^-1 at 50^@C and 7.02xx...
Text Solution
|
- Half of a substance ts consumed tn 40 min. When the quantity of substa...
Text Solution
|
- The following data are for the reaction, A+Brarr products
Text Solution
|
- The following data are for the reaction, A+Brarr products
Text Solution
|
- A+2B to 3C+2D The rate of disappearance of B is 1 xx 10^-2 mol lit^-1...
Text Solution
|
- A+2B to 3C+2D The rate of disappearance of B is 1 xx 10^-2 mole lit^-...
Text Solution
|
- A second order reaction in which both the reactants have same concentr...
Text Solution
|
- Half fife of a first order reaction is 60 min. How long will it take t...
Text Solution
|
- A general reaction A(g)+B(g)rarr C(g) +D(I) proceed in a container of ...
Text Solution
|
- A two litre vessel contains 4 moles of N2O5. On heating to 100^@C, N2O...
Text Solution
|