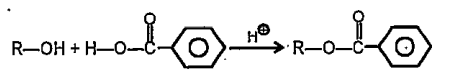
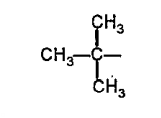
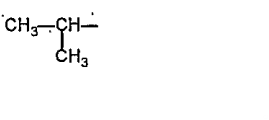A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Convert Benzoic acid to Aniline.
Text Solution
|
- Among the following carboxylic acids, the one which undergoes acid cat...
Text Solution
|
- Rate of the reaction faster when R is
Text Solution
|
- The order of reactivity of methyl alcohol (I) ,isopropyl alcohol (II) ...
Text Solution
|
- In the given reaction correct order of reactivity of HX in decreasing ...
Text Solution
|
- An unknown polyhydroxy compound (A) (molar mass=180) on acylation give...
Text Solution
|
- Convert Nitromethane to Dimethylamine.
Text Solution
|
- Convert Aniline to 2, 4, 6-Tribromofluorobenzene
Text Solution
|
- Convert Benzyl Chloride to 2-phenylethanamine
Text Solution
|
- Convert Aniline to p-bromoaniline
Text Solution
|
- Which alkenes would you except to the major product of the following d...
Text Solution
|
- Convert Benzamide to toluene.
Text Solution
|
- ?
Text Solution
|
- ?
Text Solution
|
- Convert Chlorobenzene to p-chloroaniline.
Text Solution
|
- Convert Aniline to Benzyl alcohol.
Text Solution
|
- Aniline is soluble in aqueous HCl. Why?
Text Solution
|
- Which of the following alcohols form white with Lucas reagent at warmi...
Text Solution
|
- The Lucas test is used to distignuish 1^@,2^@"and"3^@ alcohols. The al...
Text Solution
|
- Convert 2-(2-Chloroethyl)-cyclohexan-1-one to 2-(3-Aminopropyl)-cycloh...
Text Solution
|