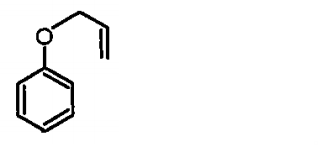
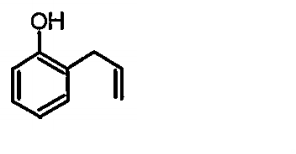
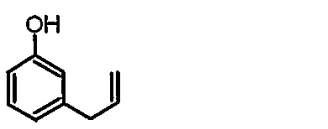
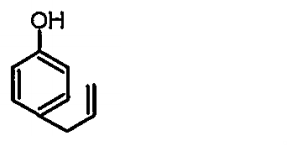
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Which of the following compounds released CO2 from NaHCO3 solution?
Text Solution
|
- Consider the following sequence of reactionsC6H5OH overset((CH3CO2)O)u...
Text Solution
|
- When phenol is refluxed with allyl bromide in acetone solution in the ...
Text Solution
|
- Which of the following alcohols gives the best yield of dialkyl ether ...
Text Solution
|
- Williamson synthesis is an example of
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which of the following ethers is/are not prepared by Williamson's synt...
Text Solution
|
- Which of the following is correct about the ether?
Text Solution
|
- (B) turns neutral FeCl3 violet. (B) and ( C) are respectively
Text Solution
|
- The products are respectively
Text Solution
|
- ProductsP-1 and P2 respectively are
Text Solution
|
- For the reaction The product obtained is
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- x moles of of HI is consumed. The value of x is
Text Solution
|
- Consider the following reactions and choose the correct answer
Text Solution
|
- In the reaction , the products is
Text Solution
|
- Convert Ethanoic acid to propanoic acid.
Text Solution
|
- (I)1,2-dihydroxybenzene (II)1,3-dihydroxybenzene (III)1,4-hydroxybenz...
Text Solution
|
- Product Z of above reaction is
Text Solution
|
- 0.092 g of a compound with the molecular formula C3H8O3 on reaction wi...
Text Solution
|