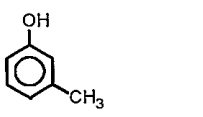
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Identify (X) and (Y) in the following reaction sequence.
Text Solution
|
- Compound X (Molecular formula, C5H8O) does not react appreciably with ...
Text Solution
|
- Compound (A) C7H8O is insoluble in NaHCO3 solution but dissolves in so...
Text Solution
|
- When t-butanol and n-butanol are separately treated with a few drops o...
Text Solution
|
- An optically active alcohol A(C6H10O) absorbs two mole of hydrogen mol...
Text Solution
|
- Give reason for the following in one or two sentences. "Acid catalysed...
Text Solution
|
- Convert,
Text Solution
|
- An organic liquid (A) Having pleasant odour is hydrolysed to an acid (...
Text Solution
|
- Identify (X) and (Y) in the following reaction sequence.
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- Give Chemical equation for: Reaction of Bromine in CS2 with phenol.
Text Solution
|
- Give Chemical Equation when: Phenol reacts in presence of Dil.HNO3.
Text Solution
|
- Give Chemical Equation for: Treating phenol with chloroform in presenc...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- Convert: Propene to propan-2-ol
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|
- This question has Statement I and Statement II. Of the four choices gi...
Text Solution
|