Similar Questions
Explore conceptually related problems
Recommended Questions
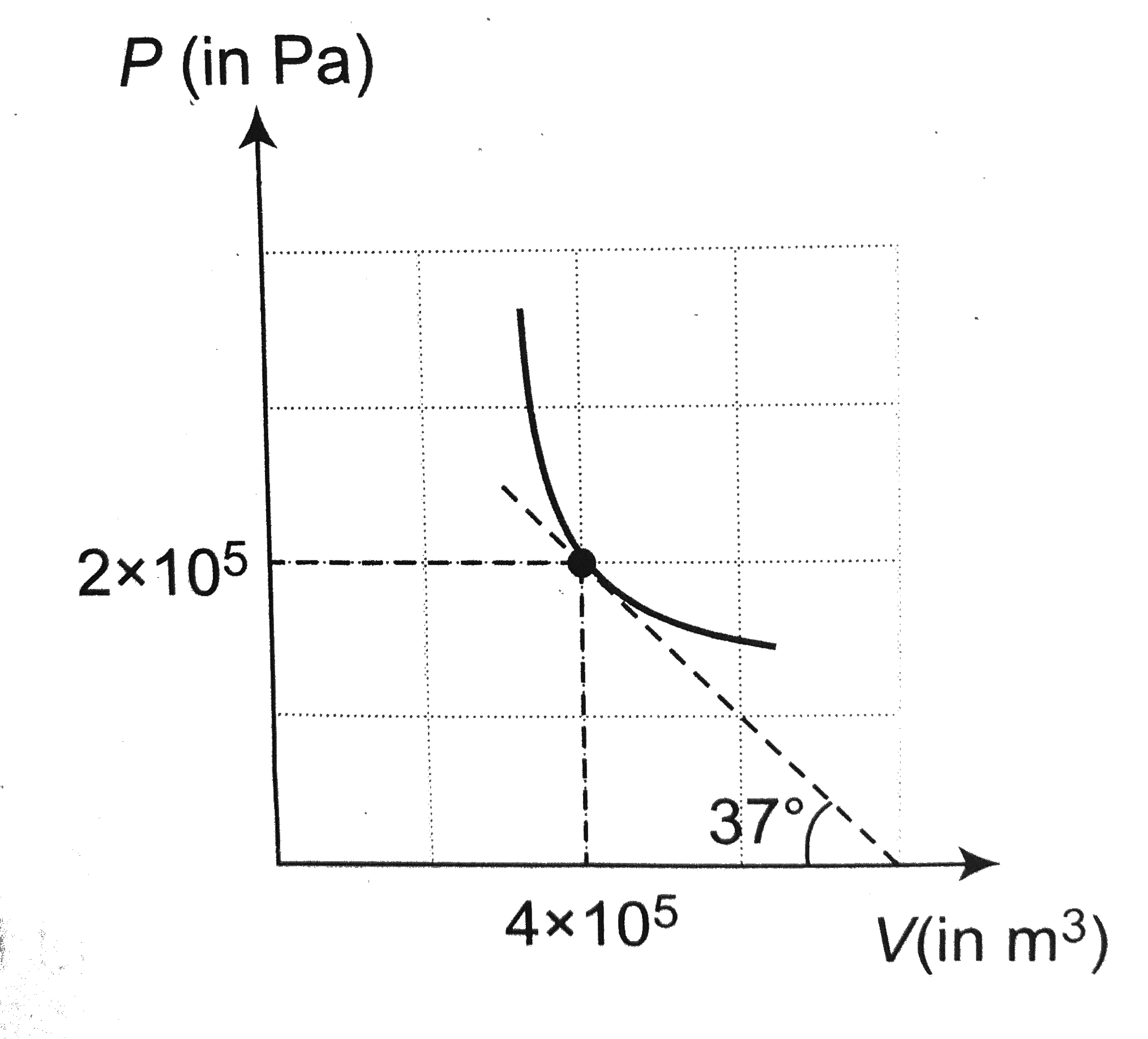
- P-V graph for an ideal gas undergoing polytropic process PV^(m) = cons...
Text Solution
|
- A certain ideal gas undergoes a polytropic process PV^(n) = constant s...
Text Solution
|
- P-V graph for an ideal gas undergoing polytropic process PV^(m) = cons...
Text Solution
|
- For an ideal gas the ratio of specific heats is (C(p))/(C(v)) = gamma ...
Text Solution
|
- For polytropic process PV^(n) = constant, molar heat capacity (C(m)) o...
Text Solution
|
- Figure demonstrates a polytropic process (i.e.PV^(n) = constant ) for ...
Text Solution
|
- An ideal monatomic gas undergoes process PV^(1.25) = constant .Then
Text Solution
|
- For polytropic process PV^(n) = constant, C(m) (molar heat capacity) o...
Text Solution
|
- For polytropic process PV^(n) = constant, C(m) (molar heat capacity) o...
Text Solution
|