Similar Questions
Explore conceptually related problems
Recommended Questions
- In the different reactions, involving a angle reactant in each case, a...
Text Solution
|
- In the different reactions, involving a angle reactant in each case, a...
Text Solution
|
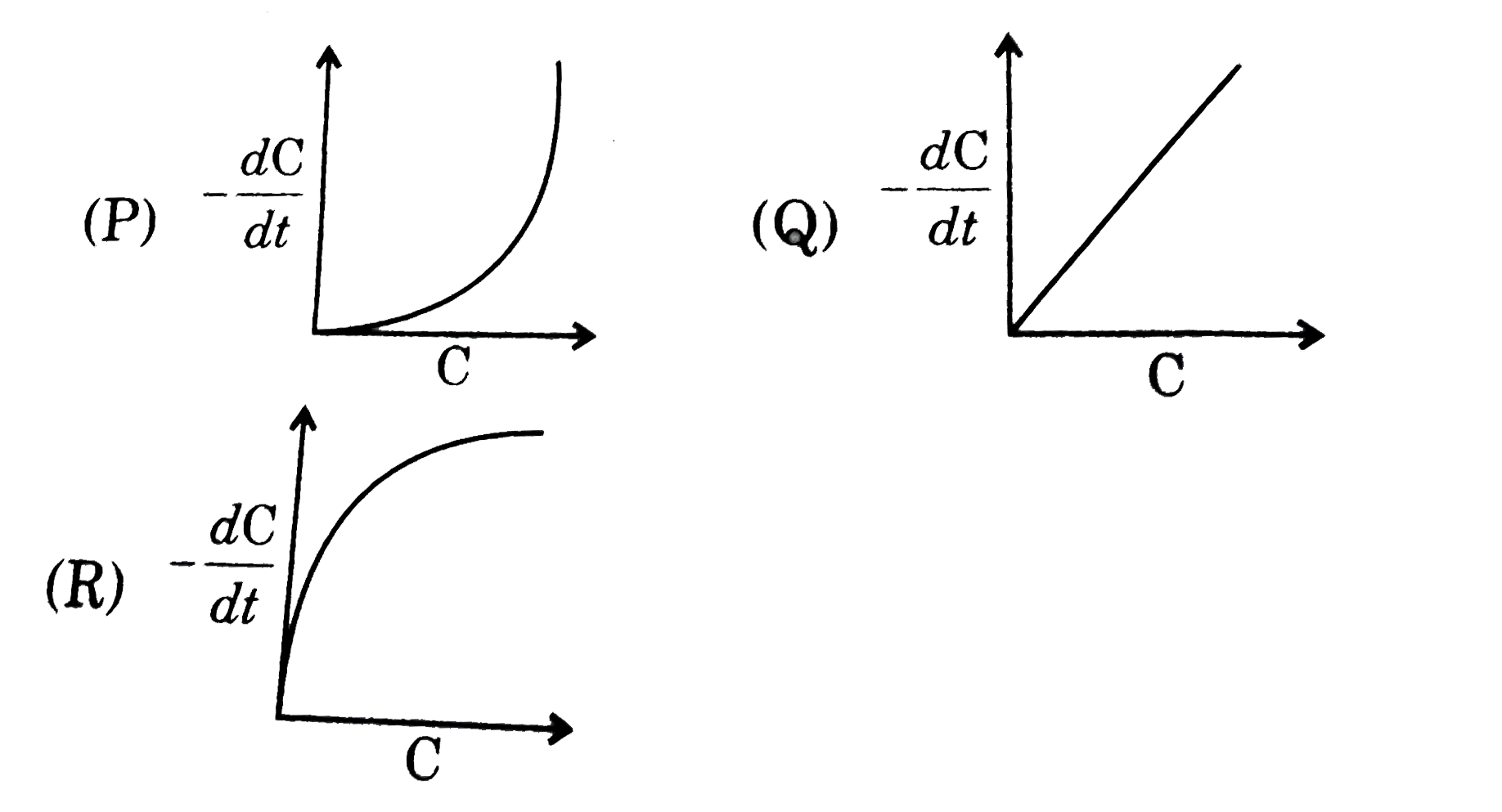
- For a zero order reaction a plot of rate (along Y-axis) and concentrat...
Text Solution
|
- For a zero order reaction the plot of concentration of reactant versus...
Text Solution
|
- The concentrations of the reactant A in the reaction A to B at differe...
Text Solution
|
- For the reaction 2A+BrarrC, the values of initial rate at different r...
Text Solution
|
- The given plots represent the variation of the concentration of a reac...
Text Solution
|
- Plot of rate of a reaction against concentration of reactant gives a s...
Text Solution
|
- plot a graph of reaction -rate vs concentration of the reactant for a ...
Text Solution
|