Similar Questions
Explore conceptually related problems
Recommended Questions
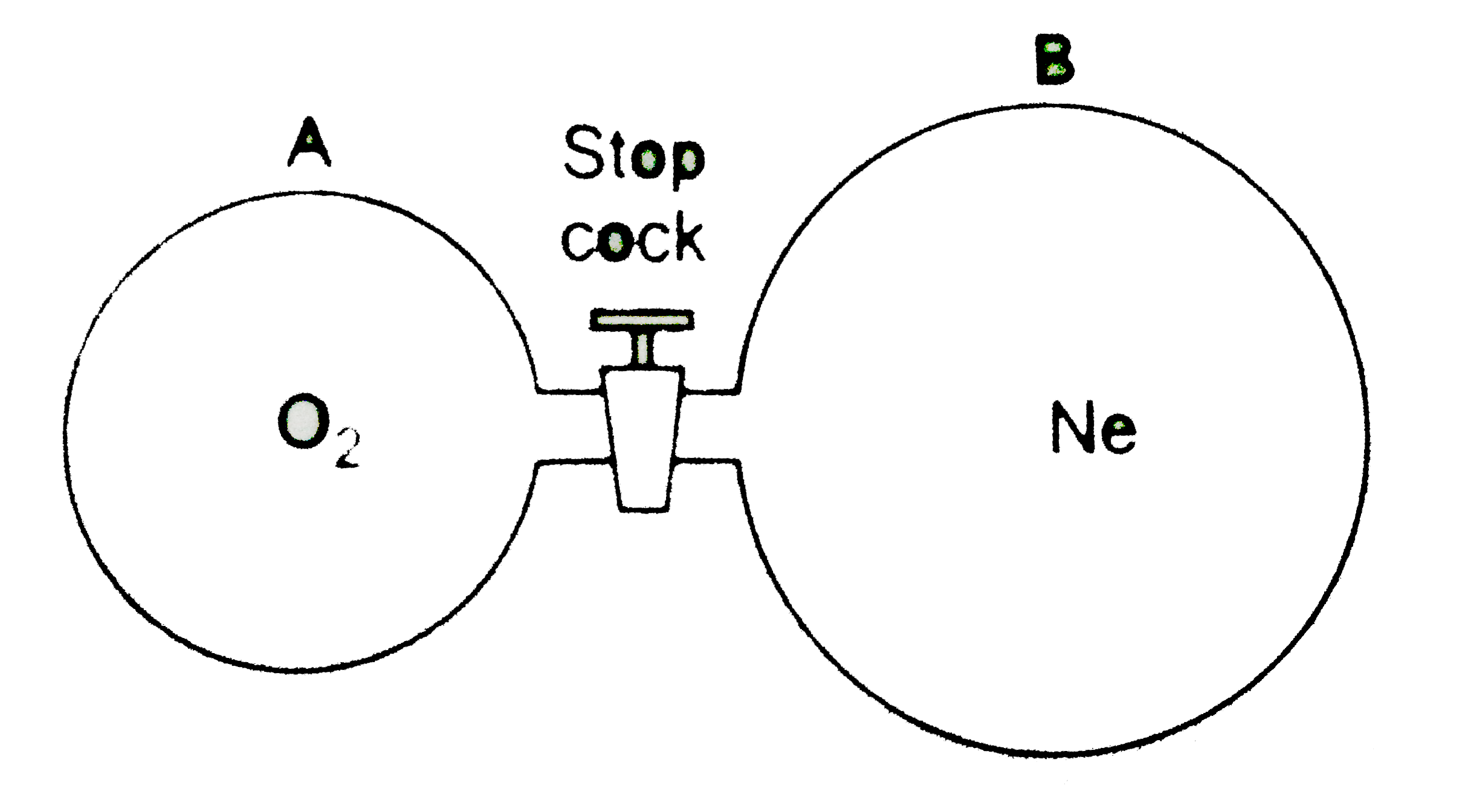
- Initially bulb "a" contained oxygen gas at 27^(@)C and 950 mm of Hg an...
Text Solution
|
- Consider the adjacent diagram. Initially, flask A contained oxygen gas...
Text Solution
|
- Consider the adjacent diagram. Initially, flask A contained oxygen gas...
Text Solution
|
- Consider the adjacent diagram. Initially, flask A contained oxygen gas...
Text Solution
|
- At 27^(@)C a gas under a pressure of 750 mm of Hg occupies a volume of...
Text Solution
|
- Initially bulb "a" contained oxygen gas at 27^(@)C and 950 mm of Hg an...
Text Solution
|
- A gas bulb containing air is connected to an open limb manometer at 27...
Text Solution
|
- A evacuated bulb of unknown volume is filled with H(2) gas at room tem...
Text Solution
|
- एक गैस का नमूना 27^(@)C तथा 740 मिमी Hg दाब पर 100 मिली स्थान घेरता ह...
Text Solution
|