Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-PRACTICAL WORK-REVIEW QUESTIONS
- State what you observe when a piece of moist blue litmus paper is plac...
Text Solution
|
- For the elements sodium and phosphorus, state the following the form...
Text Solution
|
- For the elements sodium and phosphorus, state the following The phys...
Text Solution
|
- For the elements sodium and phosphorus, state the following the natu...
Text Solution
|
- State what you observe when a piece of moist blue litmus paper is plac...
Text Solution
|
- Write correctly a balanced equation for the following “word equation":...
Text Solution
|
- What happens if carbon dioxide is bubbled into a suspension of calcium...
Text Solution
|
- Give a chemical test to distinguish between the following pairs of com...
Text Solution
|
- Complete the following "word" equation : lime water + carbon dioxide t...
Text Solution
|
- Give reasons for: 'It is dangerous to sleep in a closed room in which ...
Text Solution
|
- Give the name of an acid salt found in "Health Salts".
Text Solution
|
- Name two important processes which generate or release carbon dioxide ...
Text Solution
|
- Name two processes which remove carbon dioxide from the atmosphere.
Text Solution
|
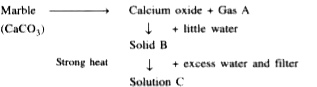
- Study the reaction scheme below and then answer which follow: Giv...
Text Solution
|
- Study the reaction scheme below and then answer which follow: Giv...
Text Solution
|
- Study the reaction scheme below and then answer which follow: Giv...
Text Solution
|
- Study the reaction scheme below and then answer which follow: On ...
Text Solution
|
- Explain the following: A white crust forms on the surface of lime wa...
Text Solution
|
- Give the biological importance of carbon dioxide dissolved in water.
Text Solution
|
- State your observations and give balanced equations of the reactions w...
Text Solution
|