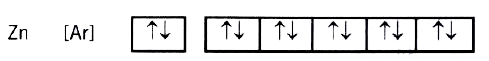A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
ICSE|Exercise True Or False Type Questions|19 VideosSTRUCTURE OF ATOM
ICSE|Exercise Fill in the Blanks Type Questions|20 VideosSTRUCTURE OF ATOM
ICSE|Exercise Essay (Long Answer) Type Questions|15 VideosSTATES OF MATTER : GASES AND LIQUIDS
ICSE|Exercise NCERT TEXT-BOOK EXERCISES (With Hints and Solutions)|23 VideosTHE s - BLOCK ELEMENTS
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES (WITH HINTS AND SOLUTIONS)|55 Videos
Similar Questions
Explore conceptually related problems
ICSE-STRUCTURE OF ATOM-Objective (MCQ) TYPE Questions
- The total number of neutrons in dipositive zinc ions with mass number ...
Text Solution
|
- A p-orbital can accommodate
Text Solution
|
- Which of the following is not correct for electronic distribution in t...
Text Solution
|
- Which of the following electron transitions will require the largest a...
Text Solution
|
- A 200 g cricket ball is thrown with a speed of 3 xx 10^3 cm/sec, what ...
Text Solution
|
- For the energy levels in an atom which one of the following statements...
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorb a photo...
Text Solution
|
- Spectrum produced due to transition of an electron from M to L shell i...
Text Solution
|
- Energy of the third orbit of Bohr's atom is
Text Solution
|
- If the speed of the electron in the Bohr's first orbit is x, then spee...
Text Solution
|
- An ion has 18 electrons in the outermost shell , it is
Text Solution
|
- Which of the following statement(s) is not correct?
Text Solution
|
- The electrons, identified by quantum numbers n and l(i) n=4,l=1 (ii) n...
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- The number of nodal planes in a p(x) orbital is
Text Solution
|
- The electronic configuration of an element is 1s^2 2s^2 2p^6 3s^2 3p^6...
Text Solution
|
- Which of the following statements regarding the electron spin +1/2 and...
Text Solution
|
- The speed of a 200 g golf ball is 5.0 metre per hour. The wavelength o...
Text Solution
|
- Energy of H-atom in the ground state is -13.6 eV, hence energy in the ...
Text Solution
|
- Uncertainty in position of a particle of 25 g in space is 10^(-5) m. H...
Text Solution
|