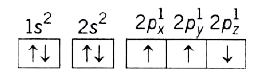
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
ICSE|Exercise True Or False Type Questions|19 VideosSTRUCTURE OF ATOM
ICSE|Exercise Fill in the Blanks Type Questions|20 VideosSTRUCTURE OF ATOM
ICSE|Exercise Essay (Long Answer) Type Questions|15 VideosSTATES OF MATTER : GASES AND LIQUIDS
ICSE|Exercise NCERT TEXT-BOOK EXERCISES (With Hints and Solutions)|23 VideosTHE s - BLOCK ELEMENTS
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES (WITH HINTS AND SOLUTIONS)|55 Videos
Similar Questions
Explore conceptually related problems
ICSE-STRUCTURE OF ATOM-Objective (MCQ) TYPE Questions
- If n=6, the correct sequence for filling of electrons will be
Text Solution
|
- The correct set of four quantum numbers for the valence elections of r...
Text Solution
|
- What is the maximum number of orbitals that can be identified with the...
Text Solution
|
- Calculate the energy in joule corresponding to light of wavelength 45 ...
Text Solution
|
- Energy of an electron is given by E=-2.178'10^(-18)J Wavelength of...
Text Solution
|
- The electrons, identified by quantum numbers n and l(i) n=4,l=1 (ii) n...
Text Solution
|
- A gas absorbs photon of 355 nm and emits at two wavelengths. If one of...
Text Solution
|
- The frequency of light emitted for the transition n =4 to n =2 of He^+...
Text Solution
|
- The energy required to break one mole of Cl-Cl bonds in Cl2 is 242 kJ ...
Text Solution
|
- Which one is the wrong statement ?
Text Solution
|
- The radius of the second Bohr orbit for hydrogen atom is [Planck's con...
Text Solution
|
- The most abundant elements by mass in the body of a healthy human adul...
Text Solution
|
- Which one is wrong statement?
Text Solution
|
- 4d, 5p, 5f and 6p orbitals are arranged in the order of decreasing ene...
Text Solution
|
- Which of the following series of transitions in the spectrum of hydrog...
Text Solution
|
- In which of the following, energy of 2s orbital is minimum
Text Solution
|
- Which one of the following about an electron occupying the 1s orbital ...
Text Solution
|
- The size of the iso-electronic species Cl^(-), Ar and Ca^(2+) is affec...
Text Solution
|
- The quantum number of four electrons are given below: n=4,l=2,m(l)=-...
Text Solution
|
- If p is the momentum of the fastest electron ejected from a metal surf...
Text Solution
|