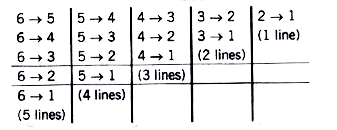Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-STRUCTURE OF ATOM-NCERT Textbook Exercises
- what is the wavelength of light emitted when the electron in a hydroge...
Text Solution
|
- how much energy is required to ionise a H atom if the electron occupie...
Text Solution
|
- what is the maximum number of emission lines when the exectied electro...
Text Solution
|
- the energy associated with the first orbit in the hydrogen atom is -2....
Text Solution
|
- calculate the wavenumber for the longest wavelength transition in the ...
Text Solution
|
- what is the energy in joules, required to shift the electron of the hy...
Text Solution
|
- The electron energy in hydrogen atom is given by E(n)=-(2.18 xx 10^(-1...
Text Solution
|
- Calculate the wavelength of an electron moving with a velocity of 2.05...
Text Solution
|
- the mass of an electron is 9.1 xx 10^(-31) kg . If its K.E. is 3.0xx...
Text Solution
|
- Which of the following are isoelectronic species i.e, those having the...
Text Solution
|
- (i) Write the electronic configurations of the following ions: (a) H...
Text Solution
|
- What is the lowest value of n that allows g orbital to exist?
Text Solution
|
- An electron is in one of the 3d-orbitals. What are the possible values...
Text Solution
|
- an atom of an element contains 29 electrons and 35 neutrons . Dedue (i...
Text Solution
|
- Give the number of electrons in the species H2^+. H2, and O(2)^+.
Text Solution
|
- An tomic obital has n=3 , what are the possible values of l and m(l) ...
Text Solution
|
- Using s,p,d notations, describe the orbital with the following quantum...
Text Solution
|
- Explain giving reasons, which of the following sets of quantum numbers...
Text Solution
|
- How many electrons in an atom may have the following quantum numbers? ...
Text Solution
|
- Show that the circumference of the Bohr orbit for the hydrogen atom is...
Text Solution
|