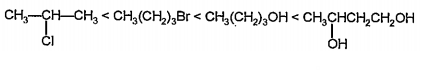Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Write structures of the products of the reaction
Text Solution
|
- Write structures of the products of the reaction
Text Solution
|
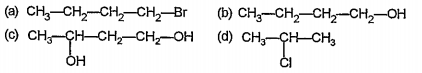
- Arrange the following in the order of increasing boiling points giving...
Text Solution
|
- Why 2-chloroethanol is more acidic than ethanol ?
Text Solution
|
- 0.436 g of acetyl derivative of a polyhydric alcohol(molecular mass=92...
Text Solution
|
- State the reaction: Benzyl ethyl ether reacts with HI.
Text Solution
|
- Why is sulfuric acid not used during the reaction of alcohols with KI?
Text Solution
|
- Write the isomers of compounds having molecular formula C4H9Br.
Text Solution
|
- Convert: Butan-1-ol to 1-iodobutane.
Text Solution
|
- Give the major product formed when each alcohol in the presence of H2...
Text Solution
|
- Give the major product formed when each alcohol in the presence of H2...
Text Solution
|
- Give the major product formed when each alcohol in the presence of H2...
Text Solution
|
- Give the major product formed when each alcohol in the presence of H2...
Text Solution
|
- Predict the major product of acid catalysed dehydration of (a) 1-meth...
Text Solution
|
- Propose a mechanism for the following reaction.
Text Solution
|
- Classify alcohols as primary and secondary alcohols and write the stru...
Text Solution
|
- Classify alcohols as primary and secondary alcohols and write the stru...
Text Solution
|
- Starting with bromobenzene and any other needed reagents, outline a sy...
Text Solution
|
- Draw the organic products in each reaction:
Text Solution
|
- Draw the organic products in each reaction:
Text Solution
|