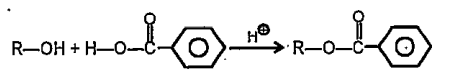
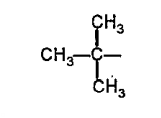
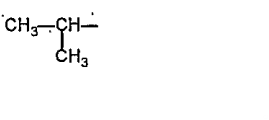A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Which of the following would be least soluble in water?
Text Solution
|
- Among the following carboxylic acids, the one which undergoes acid cat...
Text Solution
|
- Rate of the reaction faster when R is
Text Solution
|
- The order of reactivity of methyl alcohol (I) ,isopropyl alcohol (II) ...
Text Solution
|
- In the given reaction correct order of reactivity of HX in decreasing ...
Text Solution
|
- An unknown polyhydroxy compound (A) (molar mass=180) on acylation give...
Text Solution
|
- is best achieved through use of the reagent in a low temperature react...
Text Solution
|
- The compound that undergoes dehydration very easily is
Text Solution
|
- Which one of the following will readily be dehydrated in acidic medium...
Text Solution
|
- In the following reactions, The major products (A)and( C) are respec...
Text Solution
|
- Which alkenes would you except to the major product of the following d...
Text Solution
|
- The expected major product may be
Text Solution
|
- ?
Text Solution
|
- ?
Text Solution
|
- Compound (A)and (B) are respectively
Text Solution
|
- Products obtained are
Text Solution
|
- One mole of glycerol is treated with an excess of HIO4. The number of ...
Text Solution
|
- Which of the following alcohols form white with Lucas reagent at warmi...
Text Solution
|
- The Lucas test is used to distignuish 1^@,2^@"and"3^@ alcohols. The al...
Text Solution
|
- R-OHoverset(P+l2)rarroverset(AgNO2)rarr overset((i)HNO2)underset((ii)a...
Text Solution
|