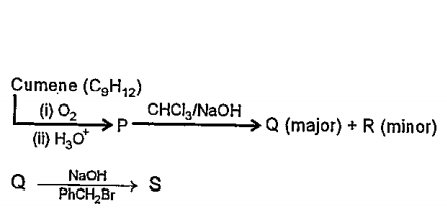
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Compound (A) molecular formula C5H12 O is optically active and is oxid...
Text Solution
|
- Predict the major product of the following reaction
Text Solution
|
- The correct statement(s) about the following reaction sequence is (are...
Text Solution
|
- The reactivity of compound Z with different halogens under appropriate...
Text Solution
|
- The acidic hydrolysis of ether (X)shown below is fastest when
Text Solution
|
- For the identification of beta naphthol using dye test, it is necessar...
Text Solution
|
- The major product(s) of the following reaction is (are)
Text Solution
|
- An unknown alcohol is treated with the "Lucas reagent" to determine wh...
Text Solution
|
- Which of the following reagents can be used to carry out the following...
Text Solution
|
- (X)+Mgoverset(dry)underset (ether) rarr (Y) overset ((i)(Z))underset((...
Text Solution
|
- Among the given geminal diols, which is/are stable with respect to the...
Text Solution
|
- Dehydration of alcohols take place more rapidly with POCl3 than with H...
Text Solution
|
- Which of the following alcohols turn(s)CrO3 inH2SO4 into green?
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which of the following alcohols respond(s) to iodoform test?
Text Solution
|
- Ethanol is less acidic than
Text Solution
|
- Phenol is less acidic than
Text Solution
|
- Which of the following compounds is/are soluble in NaHCO3?
Text Solution
|
- Reimer Tiemann introduces an aldehyde group on to the aromatic ring of...
Text Solution
|
- Reimer Tiemann introduces an aldehyde group on to the aromatic ring of...
Text Solution
|