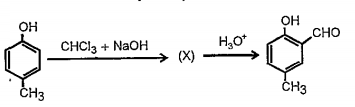
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PATHFINDER-FG-1 (ALCOHOLS, PHENOLS & ETHERS)-QUESTION BANK
- Phenol is less acidic than
Text Solution
|
- Which of the following compounds is/are soluble in NaHCO3?
Text Solution
|
- Reimer Tiemann introduces an aldehyde group on to the aromatic ring of...
Text Solution
|
- Reimer Tiemann introduces an aldehyde group on to the aromatic ring of...
Text Solution
|
- Two isomeric forms of an organic compound A, C11H13OCl readily decolou...
Text Solution
|
- Two isomeric forms of an organic compound A, C11H13OCl readily decolou...
Text Solution
|
- Match column-1 with Column-II
Text Solution
|
- A solution of sucrose (molar mass= 342g/mol) has been prepared by diss...
Text Solution
|
- Match column-1 with Column-II
Text Solution
|
- How many compounds A through G are enol tautomers of 2-butanone?
Text Solution
|
- Consider the pairs of ethers A to F shown below. To The right of each ...
Text Solution
|
- R-CH2-Ohoverset(?)rarr R -CH2-Cl Find out number of reagents that can...
Text Solution
|
- Find out number of reagents that converts 1^@ alcohol to aldehyde.
Text Solution
|
- How many moles of Hi reacts with glycerol to give 2 iodopropane?
Text Solution
|
- Compound X (Molecular formula, C5H8O) does not react appreciably with ...
Text Solution
|
- How the following transformation can be carried out (in not more than ...
Text Solution
|
- Write the structure of the major organic product expected from each of...
Text Solution
|
- Write the structure of the major organic product expected from each of...
Text Solution
|
- Indicate steps which would convert :phenol to acetophenone
Text Solution
|
- Indicate steps which would convert :acetic acid to tert-butyl alcohol
Text Solution
|