Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION-INSIDE THE ATOM-QUESTION BANK
- Write the electronic configuration of the following element: Phosphoru...
Text Solution
|
- Write the electronic configuration of the following element: Sulphur
Text Solution
|
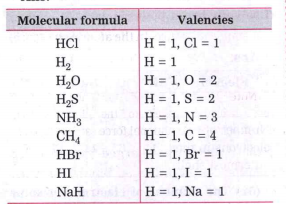
- Use the following molecular formula to determine the valencies of H, C...
Text Solution
|
- Draw suitable diagrams to show the electronic configuration of the ato...
Text Solution
|
- Diagrammatic sketch of electronic configuration of magnesium(Atomic nu...
Text Solution
|
- Diagrammatic sketch of electronic configuration of argon(Atomic number...
Text Solution
|
- What are the symbols used for the shells which accommodate the electro...
Text Solution
|
- What is the symbols and ordinal number of the innermost shell ?
Text Solution
|
- Write symbol of electron distribution in shell of fluorine atom ?
Text Solution
|
- Which is the outermost shell of fluorine atom ?
Text Solution
|
- Which is the outermost shell of sodium atom ?
Text Solution
|
- Which is the outermost shell of hydrogen atom ?
Text Solution
|
- What is meant by valency of an element ? What is the relationship betw...
Text Solution
|
- What is meant by the atomic number (Z) of an element ?
Text Solution
|
- Atomic number (Z) of some elements are given here.Write down the numbe...
Text Solution
|
- The number of electrons of some elements is given here. By using it wr...
Text Solution
|
- Why are the atomic numbers and atomic mass numbers always in whole num...
Text Solution
|
- Sulphur contains 16 protons and 16 neutrons. What would be its atomic ...
Text Solution
|
- Define: Isotopes
Text Solution
|
- State the uses of isotopes
Text Solution
|