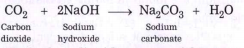Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION-METALS AND NON-METALS-QUESTION BANK
- Give Scientific reason: Ships are painted of frequent intervals
Text Solution
|
- Give Scientific reason: Gold and platinum are called noble elements
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Explain the following reactions with the help of balanced equations: ...
Text Solution
|
- Write short note on: The noble means
Text Solution
|
- Write short note on: The purity of gold
Text Solution
|
- Write short note on: Corrosion
Text Solution
|
- Write short note on: Alloys
Text Solution
|
- Distinguish between the following: Metals and Non-Metals
Text Solution
|
- Three experiments to study the process of rusting are given below. Obs...
Text Solution
|
- Three experiments to study the process of rusting are given below. Obs...
Text Solution
|
- Three experiments to study the process of rusting are given below. Obs...
Text Solution
|
- Complete the table:
Text Solution
|