Similar Questions
Explore conceptually related problems
Recommended Questions
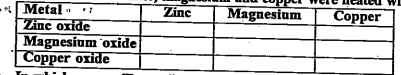
- Metallic oxide of zinc,magnesium and copper were heated with the follo...
Text Solution
|
- A students is performing displacement reactions of metals with salt so...
Text Solution
|
- जिंक, मैग्नीशियम एव कॉपर के धात्विक ऑक्साइडो को निम्न धातु के साथ गर्म...
Text Solution
|
- क्या ताम्र धातु जिंक सल्फेट के विलयन से जस्ता को विस्थापित करेगा?
Text Solution
|
- जिंक मेग्नेशियम एवं कॉपर के धात्विक ऑक्साइड को निम्न धातुओं के साथ गर्...
Text Solution
|
- क्या कारण है कि मैग्नीशियम व जिंक अम्लों से हाइड्रोजन विस्थापित ...
Text Solution
|
- Explain metal displacement redox reaction of copper sulphate solution ...
Text Solution
|
- In which case do you see the displacement action of metals such as zin...
Text Solution
|
- Metallic oxides of zinc,magnesium and copper were heated with the foll...
Text Solution
|