Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY )|4 VideosCLASSIFICATION OF ELEMENTS-THE PPERIIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|20 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES-EXERCISE
- The electronic configuration of the elements, X, Y and Z are given bel...
Text Solution
|
- State the number of valence electrons , the group number and ...
Text Solution
|
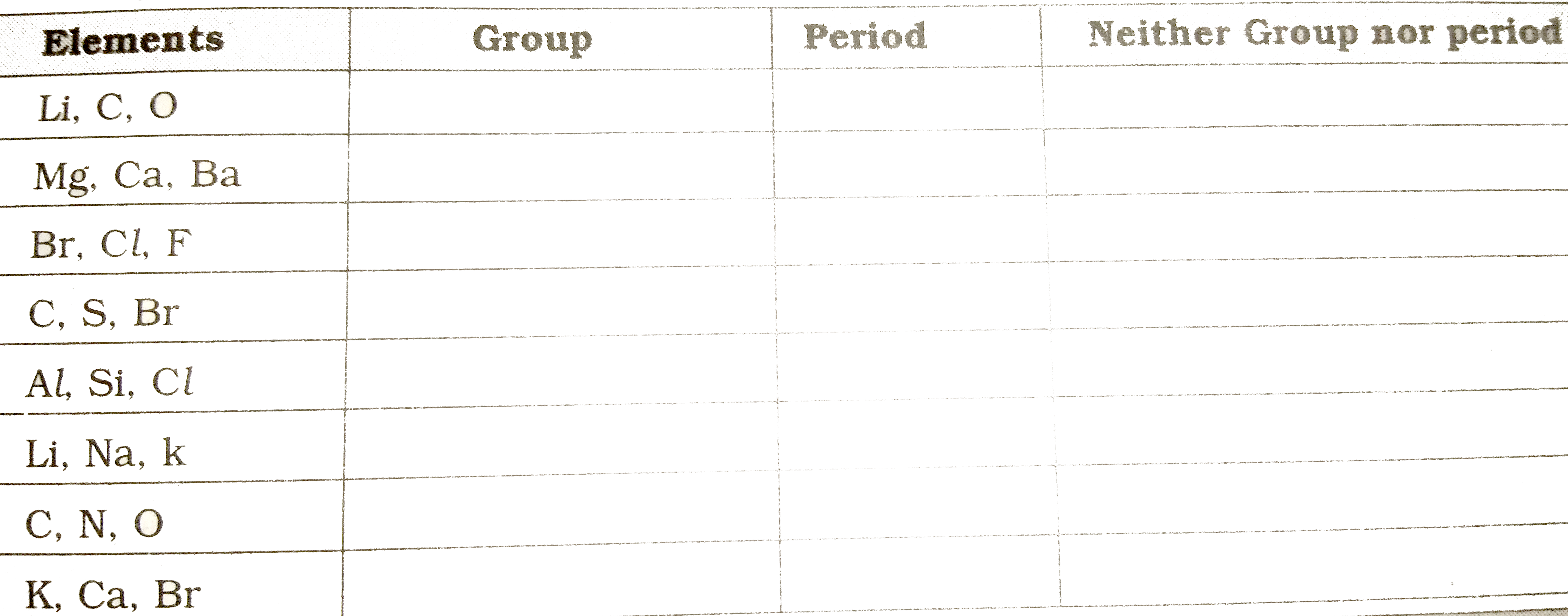
- State whether the following elements belong to a Group (G ) , Pe...
Text Solution
|
- Identify the element that has the larger atomic radius in eac...
Text Solution
|
- Identify the elements that has the lower ionization energy in e...
Text Solution
|
- How does metallic character change when we move Down a group...
Text Solution
|
- How does metallic character change when we move Down a group...
Text Solution
|
- On the basis of atomic numbers predict to which block the ele...
Text Solution
|
- Using the periodic table , predict the formula of compound form...
Text Solution
|
- An element has atomic number 19. where would you expect thi...
Text Solution
|
- How do the positions of elements in the periodic table help you...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- Number of elements present in period - 2 of the long form per...
Text Solution
|
- Nitrogen (Z=7) is the element of group V of the periodic table. Which...
Text Solution
|
- Electonic configuration of an atom is 2,8,7, . To which of the ...
Text Solution
|
- Which of the following is the most active metal ?
Text Solution
|