Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY )|4 VideosCLASSIFICATION OF ELEMENTS-THE PPERIIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|20 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES-EXERCISE
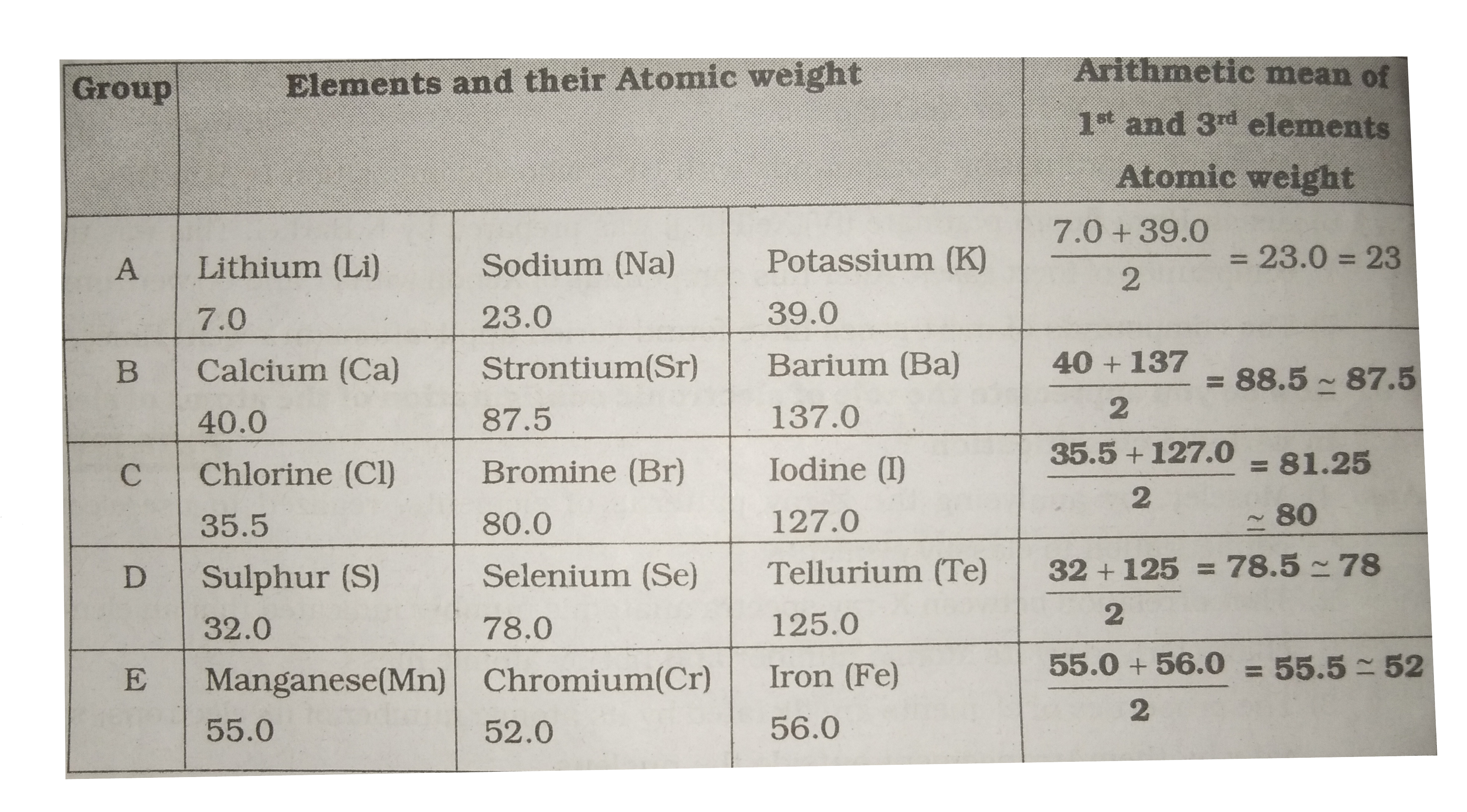
- The second period element , for example , for example 'F' has ...
Text Solution
|
- Observe the following table and Fill it . Observations ...
Text Solution
|
- Observe the following table and Fill it . Observations ...
Text Solution
|
- What is atomic number ?
Text Solution
|
- Consider the following elements of third period of modern periodic ta...
Text Solution
|
- How does the valency vary on going down a group ?
Text Solution
|
- DO the atom of an element and its ion have same size ?
Text Solution
|
- Imagine , which one in each of the following pairs is large in...
Text Solution
|
- Imagine , which one in each of the following pairs is large in...
Text Solution
|
- Which one in each of the following pairs is larger in size? S^(2-),Cl^...
Text Solution
|
- Which one in each of the following pairs is larger in size ? ...
Text Solution
|
- Which one in each of the following pairs is larger in size? C^(4-),F^(...
Text Solution
|
- Which one between Na and Na^(+) would have more size ?
Text Solution
|
- Which one between Cl and Cl^(-) would have more size ? Why ?
Text Solution
|
- A and B are two elements . The compound formed with A and B is...
Text Solution
|
- The Atomic number of an element is 35 where would you expect ...
Text Solution
|
- Why were Dobereiner , Newlands and Mendeleeff not 100 % successf...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|