Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY )|4 VideosCLASSIFICATION OF ELEMENTS-THE PPERIIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|20 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES-EXERCISE
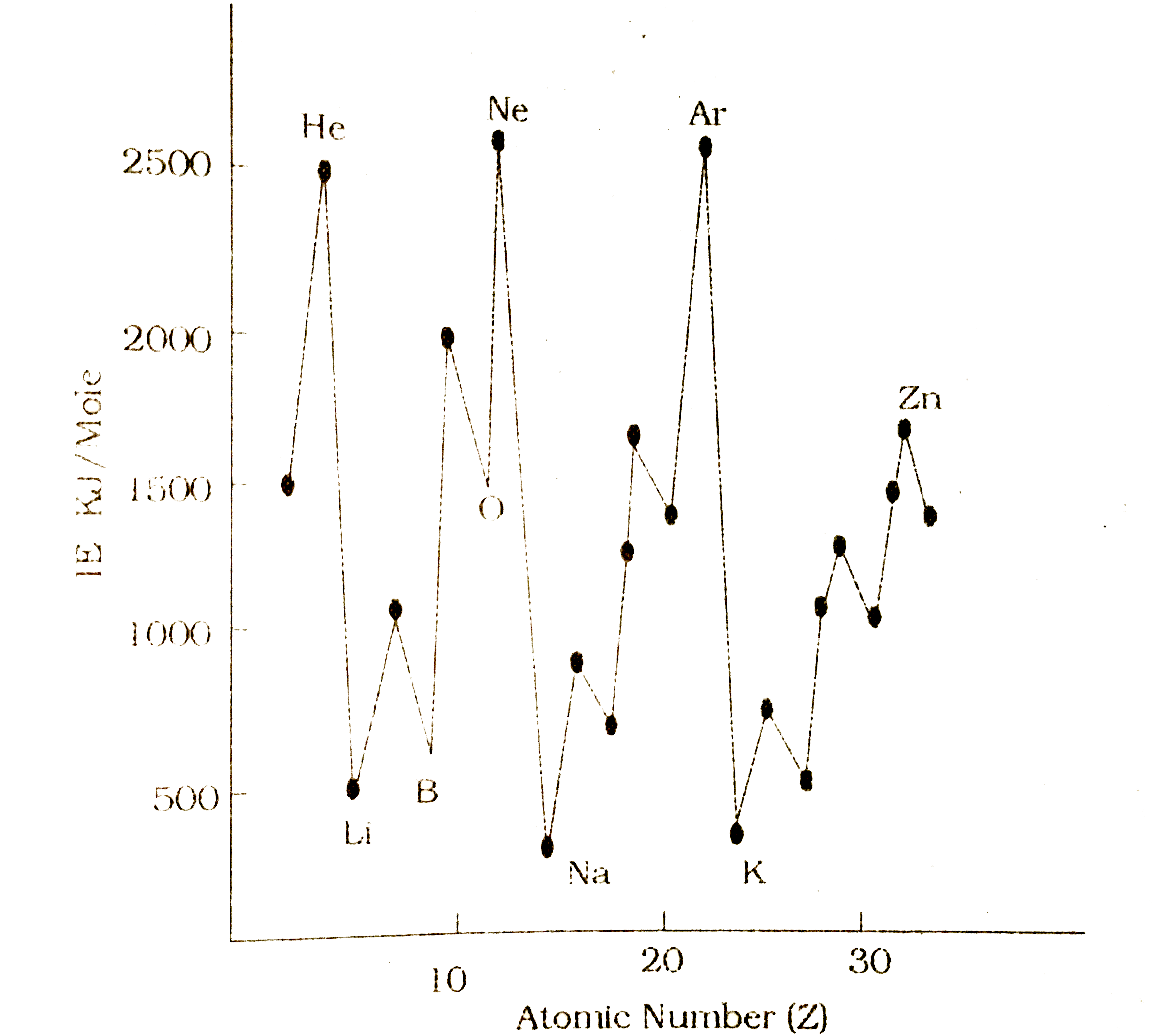
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- Ionization potential curve is the graph of atomic number versus ioniza...
Text Solution
|
- Newlands proposed the law of octaves . Mendeleeff suggested eight ...
Text Solution
|
- Why was the basis is classificatons of elements changed from t...
Text Solution
|
- Why was the basis is classificatons of elements changed from t...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- Elments in a group generally possess similar properties , but e...
Text Solution
|
- Elments in a group generally possess similar properties , but e...
Text Solution
|
- Name two elements that you would expect to have chemical prope...
Text Solution
|
- What is a group?
Text Solution
|
- What is a period in modern periodic table ?
Text Solution
|
- Mendeleev's eka-aluminium is
Text Solution
|
- Which one of the following elements has more electropositivity ?
Text Solution
|
- Number of vertical columns in the modern periodic table are (As per IU...
Text Solution
|
- A Dobereiners Triad in the following, is ........................
Text Solution
|
- Which one of the following belongs to the group of atomic weights of D...
Text Solution
|
- 11, 12, 13 and 14 are the atomic numbers of the elements Na, Mg, Al an...
Text Solution
|
- The most electronegative element is
Text Solution
|