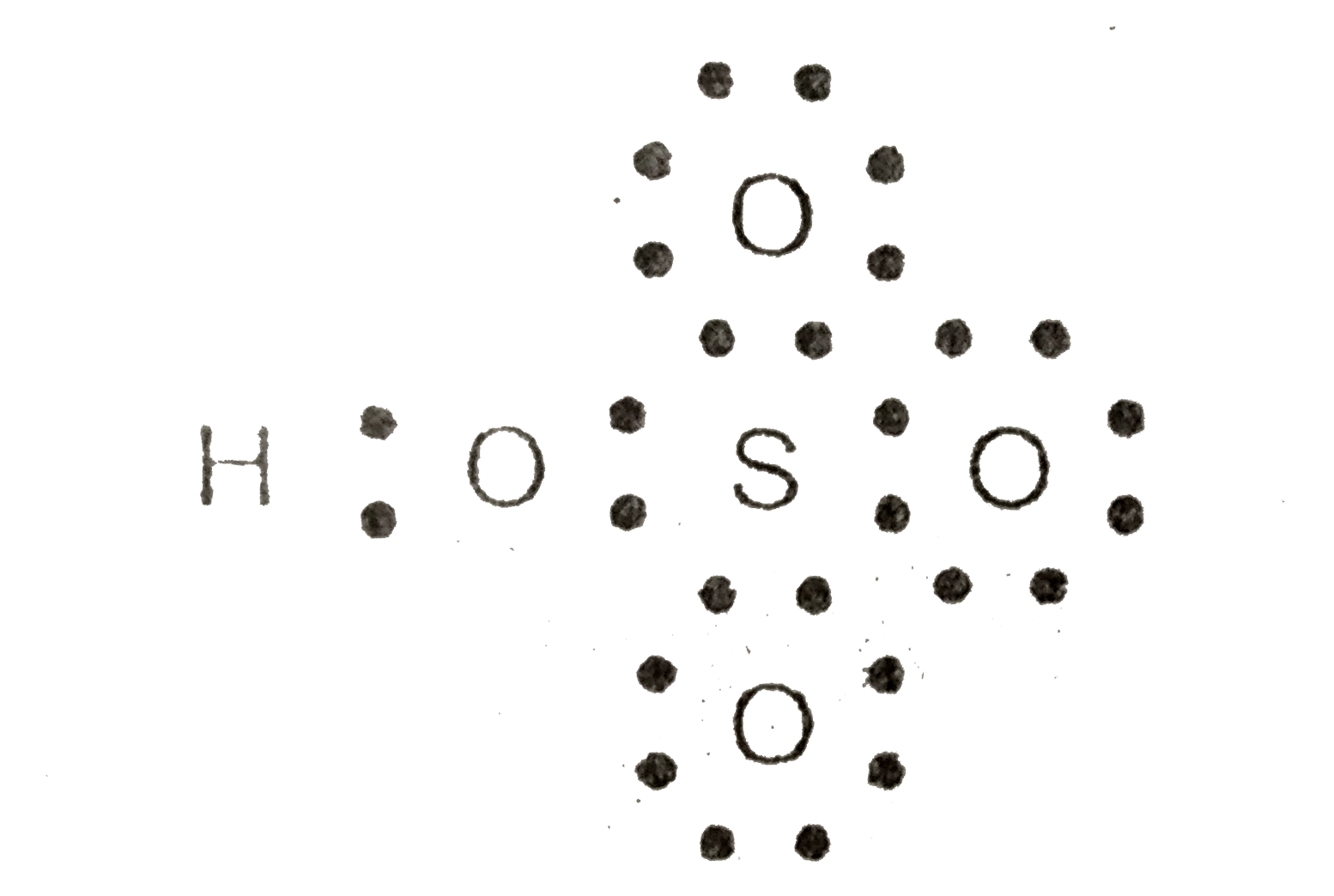Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -A Objective Type Questions (One option is correct)|60 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Assignment Section J (Aakash Challengers Questions)
- Calculate the formal charge on S in HSO(4)^(-) ion
Text Solution
|
- Shape of the compounds XeF(3)^(+) and XeF(5)^(+) are respectively
Text Solution
|
- In which of the following compounds , back bonding is possible
Text Solution
|
- The correct sequence regarding bond angle
Text Solution
|
- Correct regarding hydrogen bond strength and boiling point respectivel...
Text Solution
|
- The correct order of pair regarding nodal surface
Text Solution
|
- is NH3 a good complexing agent
Text Solution
|
- Choose the correct pair regarding dipole moment
Text Solution
|
- During oxidation , in which of the following bond order increase ?
Text Solution
|
- Choose the correct statement regarding PCl(5)
Text Solution
|
- Which of the forces are considered as shortest ranged ?
Text Solution
|