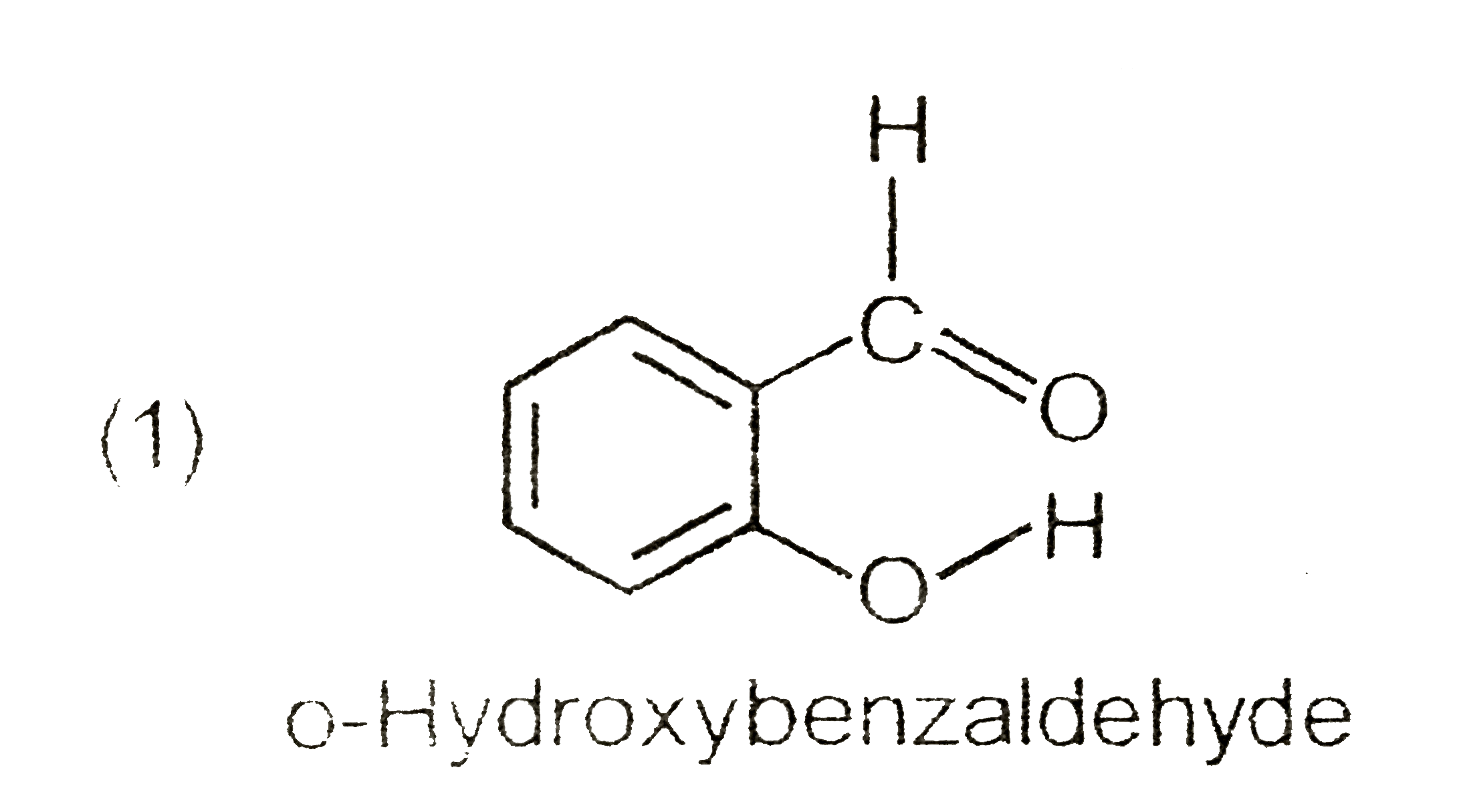
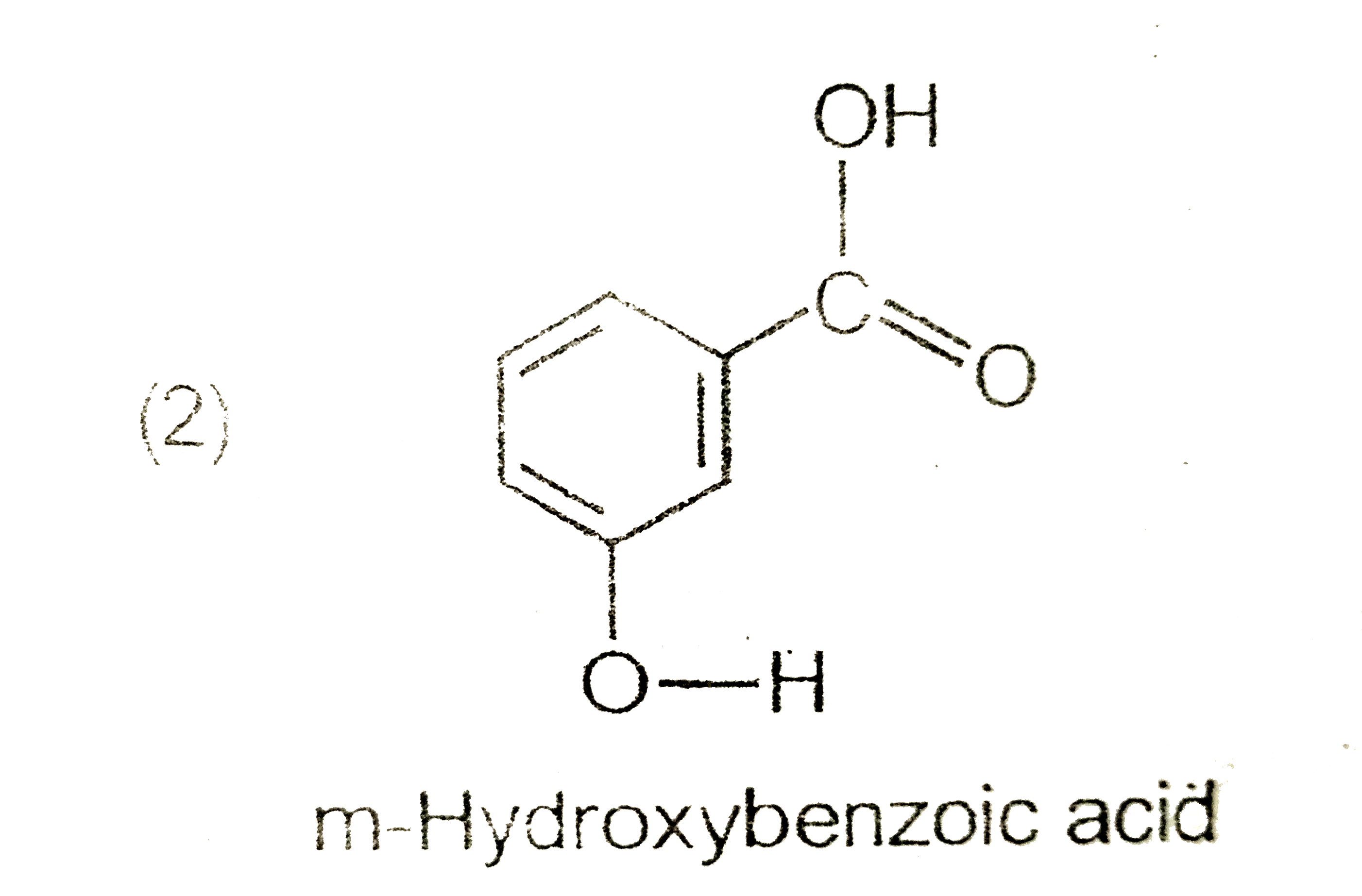
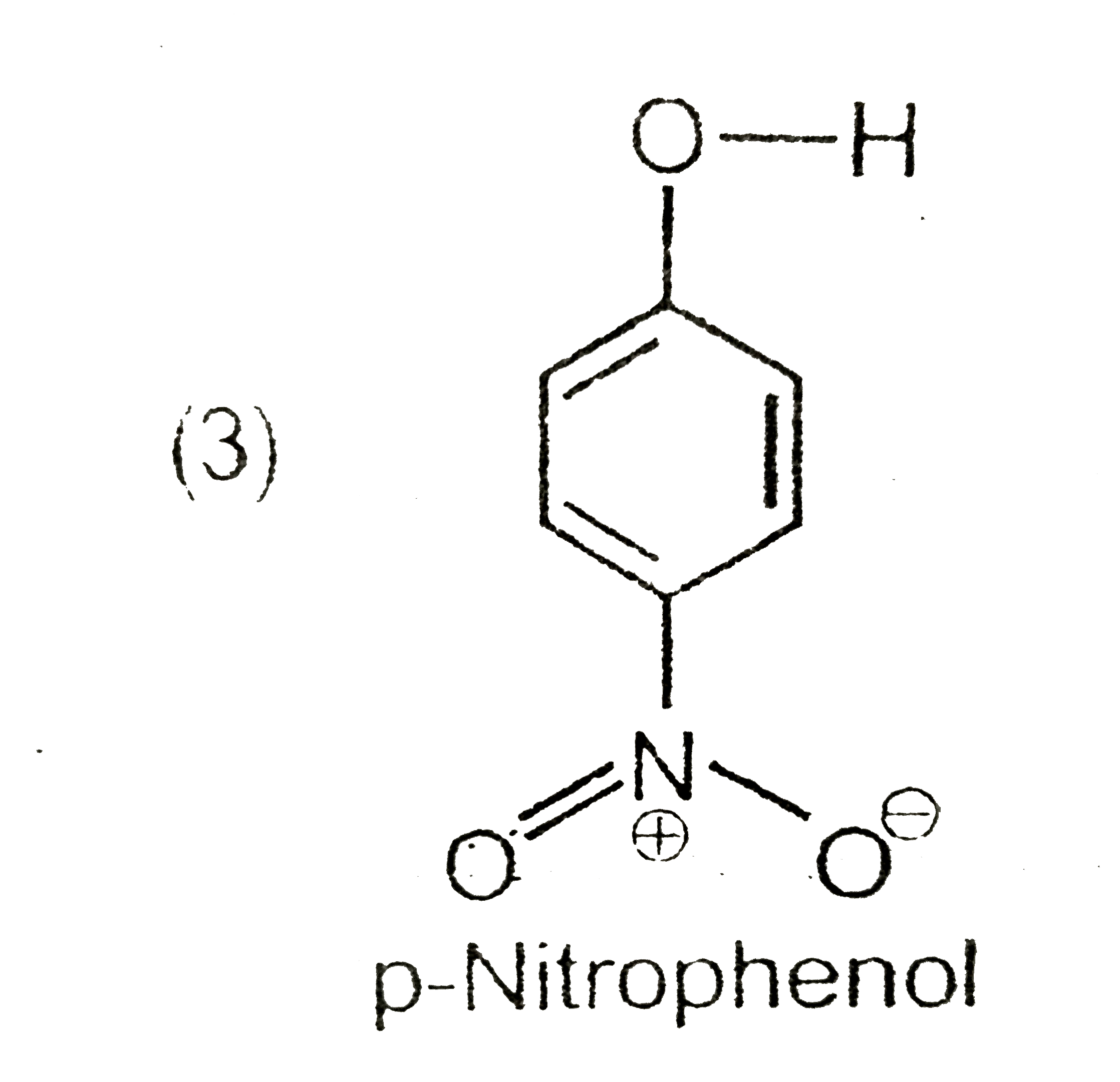
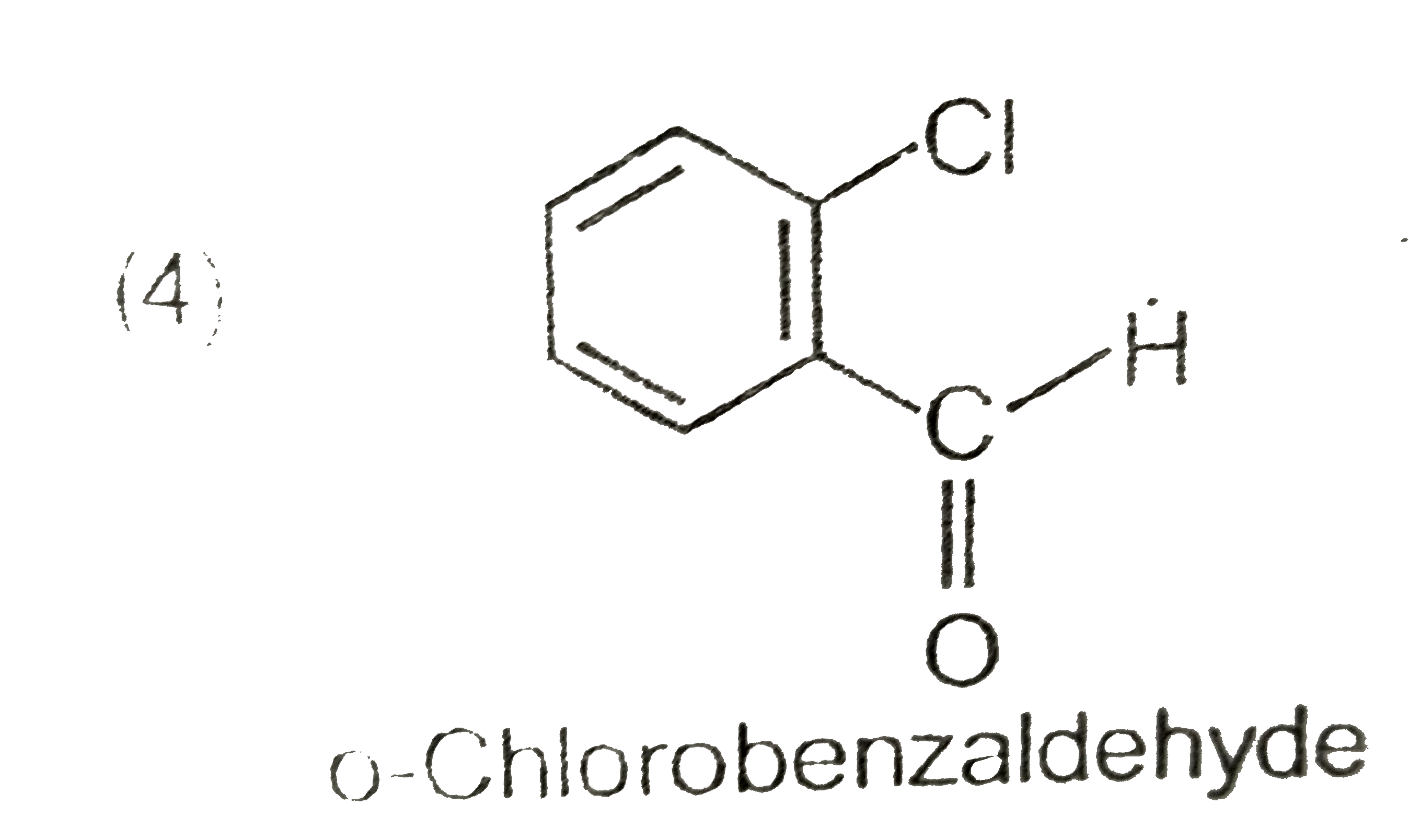
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - C Objective Type Questions(More than one options are correct )|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - D (Linked Comprehension Type Questions)|12 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -A Objective Type Questions (One option is correct)|60 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Assignment Section - B Objective Type Questions(One option is correct)
- Which of the following has zero dipole moment ?
Text Solution
|
- The compound which contains both ionic and covalent bonds is
Text Solution
|
- Which of the following species will have intramolecular hydrogen bondi...
Text Solution
|
- Which of the following pairs are iso-structural ?
Text Solution
|
- O(2) and N(2) are respectively
Text Solution
|
- Molecular shape of ClF(3) , l(3)^(-) and XeO(3) respectively are
Text Solution
|
- Hydrogen bond
Text Solution
|
- In case of XeO(2) F(2) and XeF(6) , Xe is with
Text Solution
|
- In which of the following hybridisation of underlined atom changes
Text Solution
|
- The ion that is isoelectronic with CO is
Text Solution
|
- CO(2) is isostructural with
Text Solution
|
- When NH(3) is treated with HCl, H-N-H bond angle
Text Solution
|
- Among KO2 , AlO(2)^(-) BaO2 and NO2^+ unpaired electron is present in...
Text Solution
|
- Correct order of bond angle for O-P-X P{:(O),(/),( "\" ),(X):} in ...
Text Solution
|
- Which of the following is non-linear ?
Text Solution
|
- Which of the following is electron deficient (Lewis acid ) ?
Text Solution
|
- In which of the following set of compounds , bond angle remains consta...
Text Solution
|
- Which of the following compounds have zero dipole moment ?
Text Solution
|
- Pick out the isoelectronic structures from the following (i) CH(3)^(...
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(2)^(-) is :
Text Solution
|