A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - D (Linked Comprehension Type Questions)|12 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - E (Assertion-Reason Type Questions)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - B Objective Type Questions(One option is correct)|37 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Assignment Section - C Objective Type Questions(More than one options are correct )
- CO(2) has same geometry as .
Text Solution
|
- Molecules with see-saw shape are
Text Solution
|
- Which of the following can show variable valency ?
Text Solution
|
- Molecular axis is Z axis , then which of the following combination of ...
Text Solution
|
- Isostructural group of molecules are
Text Solution
|
- In solid state PCl(5) exist as [PCl(4)]^(+) [PCl(6)]^(-). The hybridis...
Text Solution
|
- Which of the following is a correct statement ?
Text Solution
|
- Which of the following is a correct statement ?
Text Solution
|
- In which of the following pairs first partner is more soluble in water...
Text Solution
|
- Bond order increases in which of the given transitions ?
Text Solution
|
- Which of the following are correctly matched with name of bond present...
Text Solution
|
- Amongst the given structures , which are permissible resonance forms ?
Text Solution
|
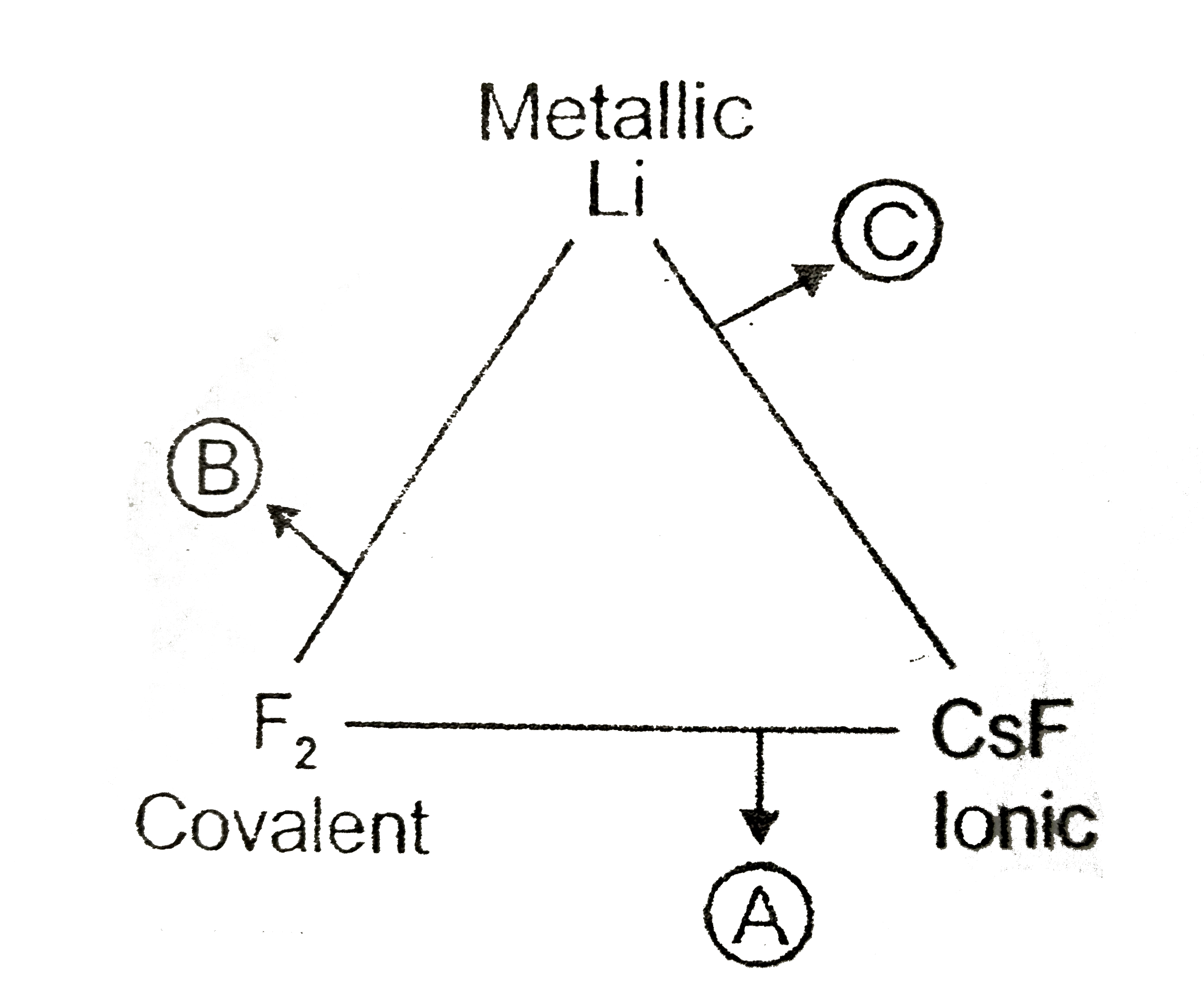
- Given below is a triangle illustrate the transition between ionic , me...
Text Solution
|