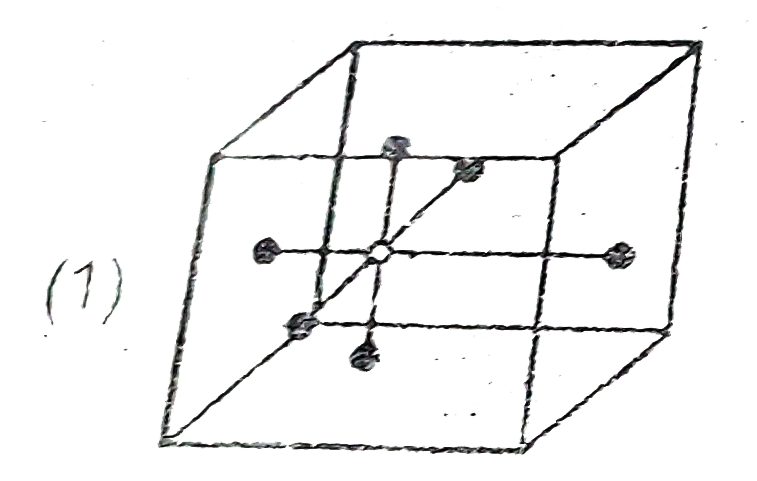
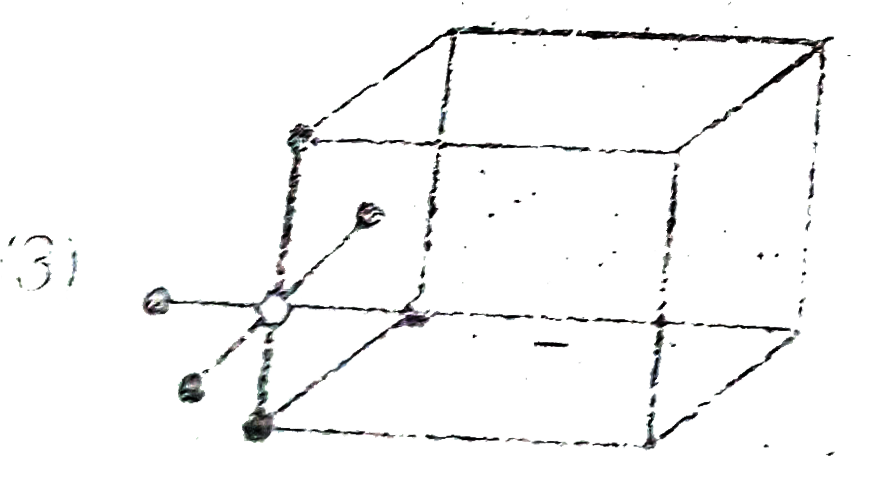
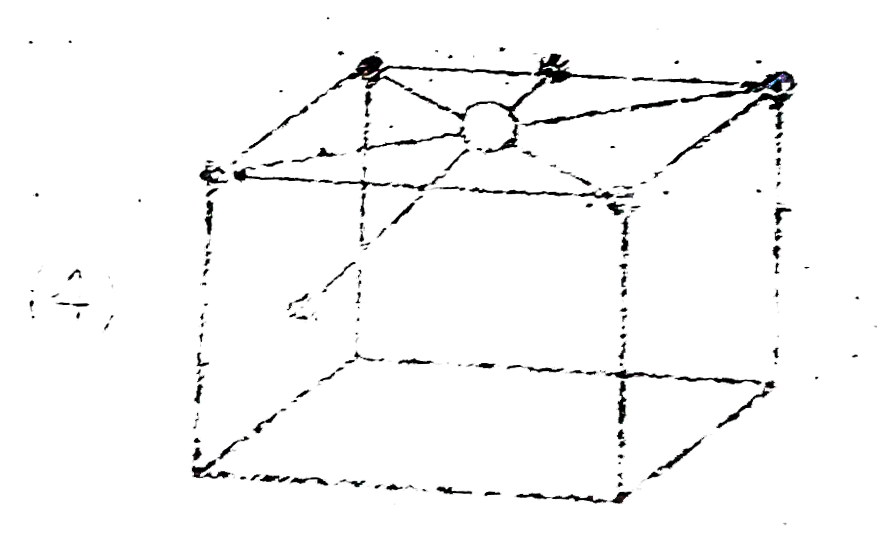
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION-D (LINKED COMPREHENSION)|9 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION-E (ASSERTION-REASON)|10 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION-B (OBJECTIVE)|27 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THE SOLID STATE -ASSIGNMENT SECTION-C (OBJECTIVE)
- Which of the following is correctly matched ?
Text Solution
|
- Which of the following is/are pseudo solid ?
Text Solution
|
- Which of the following is/are correct for fluorite structure (CaF2) ?
Text Solution
|
- Which of the following represent octahedral void ?
Text Solution
|
- Which of the following is/are true statement(s)?
Text Solution
|
- CsCl has bcc structure . If atomic mass of Cs and Cl atom is 133 and 3...
Text Solution
|
- Fe3O4 has inverse spinel structure. What is not true about this solid ...
Text Solution
|
- The density of KBr is 2.75 g cm^(-3). The length of the unit cell is 6...
Text Solution
|
- In fluorite structure of AB2
Text Solution
|
- Which of the following defects doesn't disturb stoichiometry of solid ...
Text Solution
|
- For hcp lattice which statements will be correct ?
Text Solution
|
- Which of the following is/are true statement(s)?
Text Solution
|
- What will be the distance between two nearest neighbours in primitive ...
Text Solution
|
- In the closet packing of atoms:
Text Solution
|
- In which operations , the formula of NaCl remains same ?
Text Solution
|
- CsCl structure is interchanged into NaCl structure. This can be done b...
Text Solution
|
- All spinel structures do not have
Text Solution
|