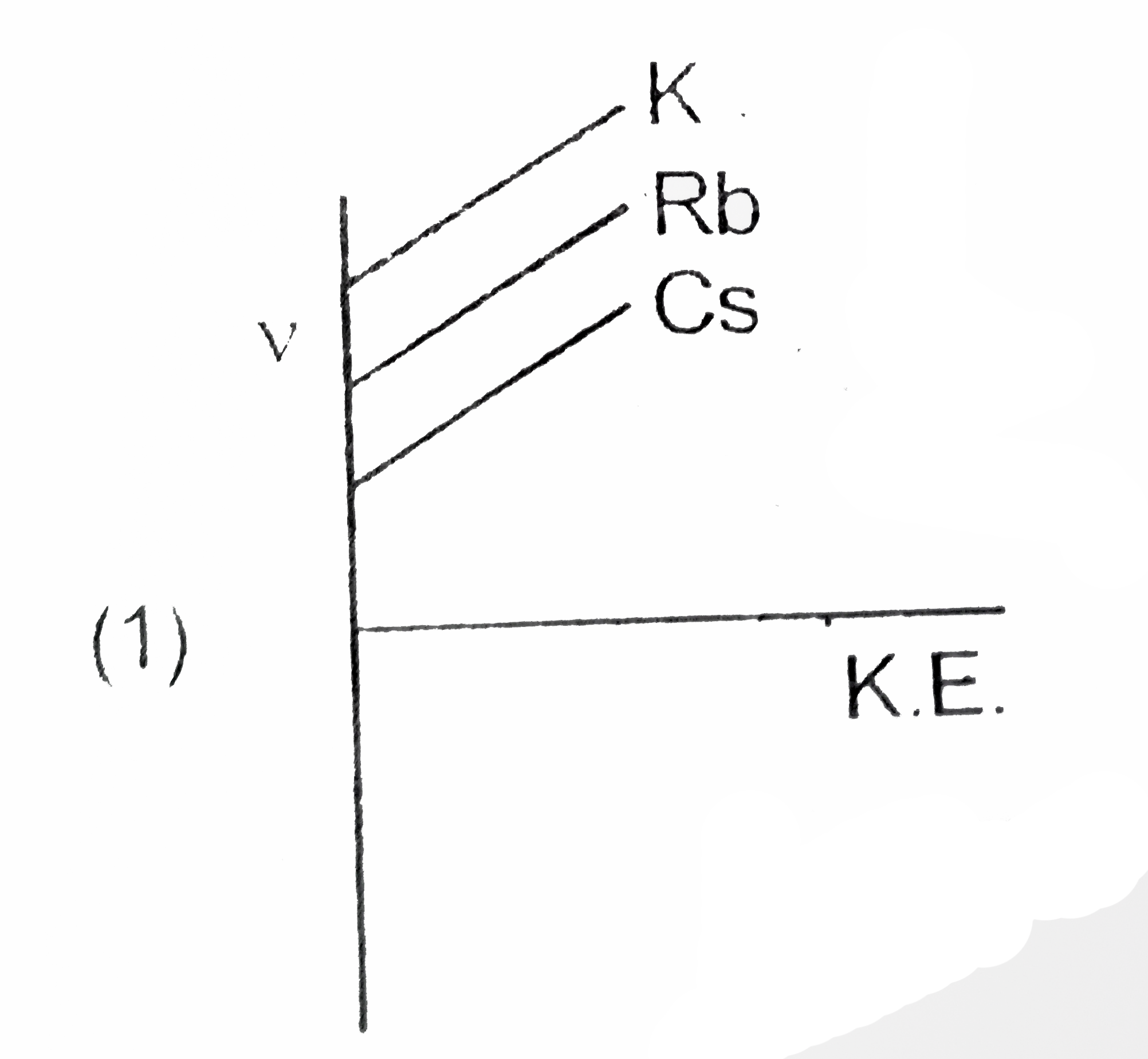
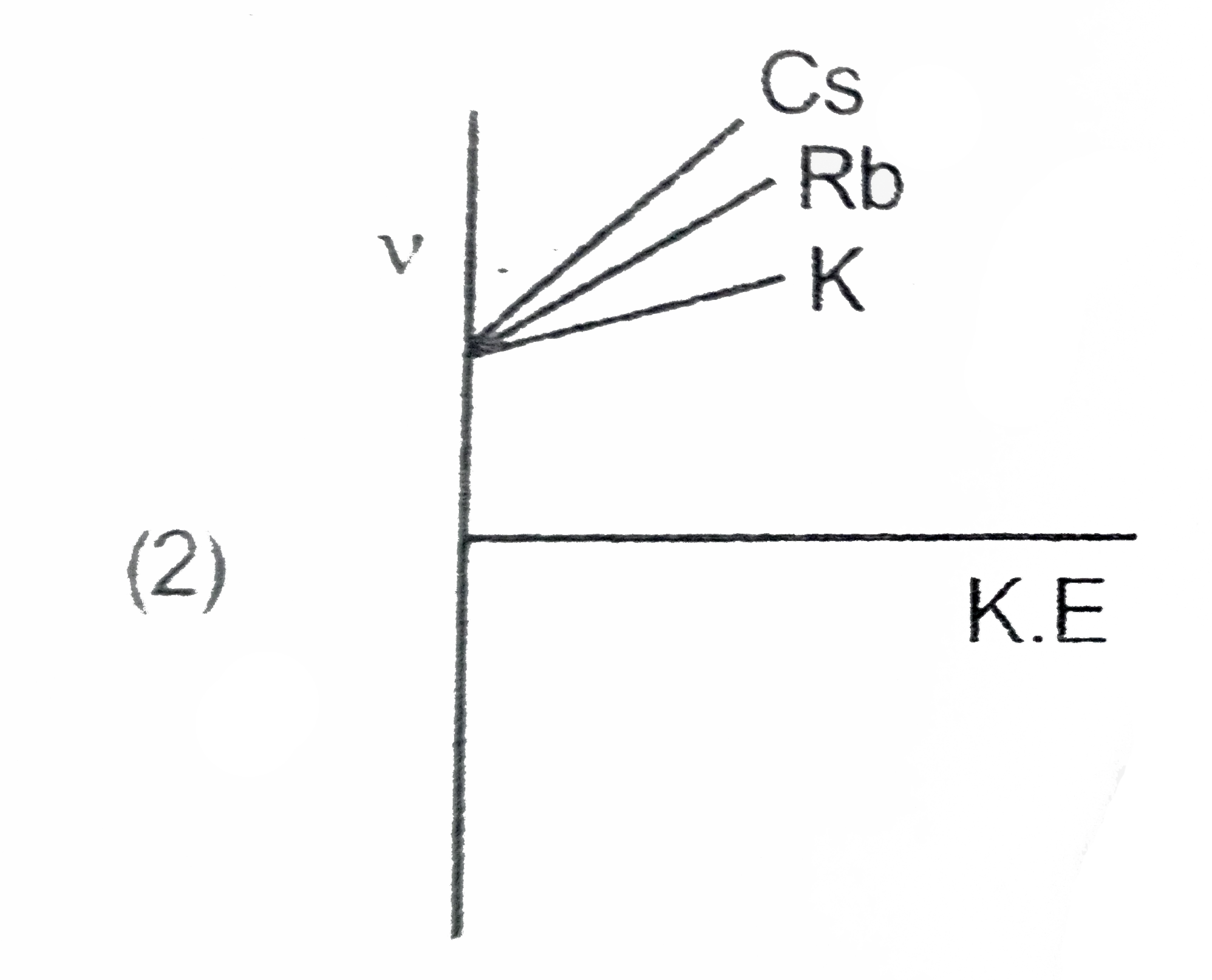
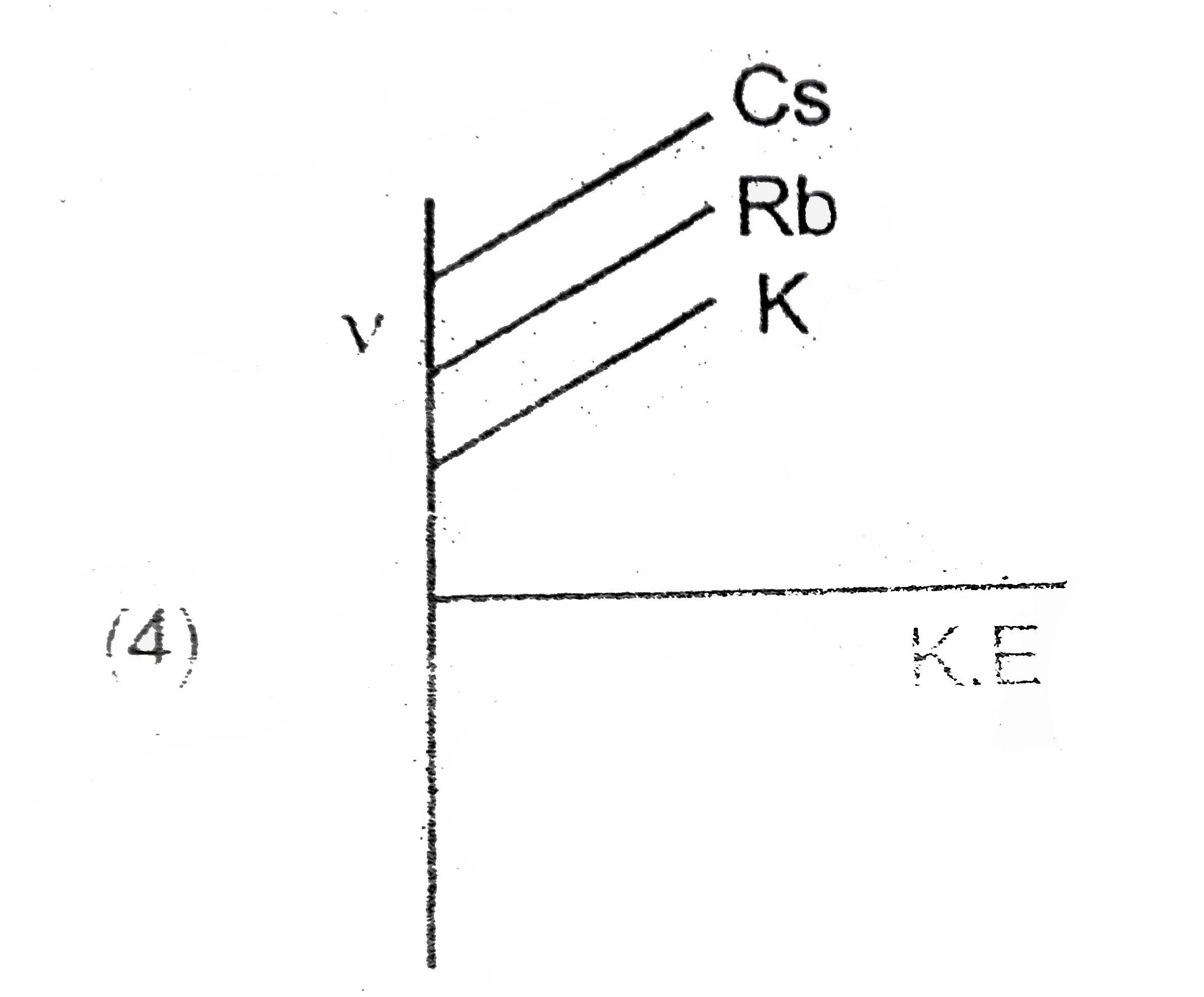
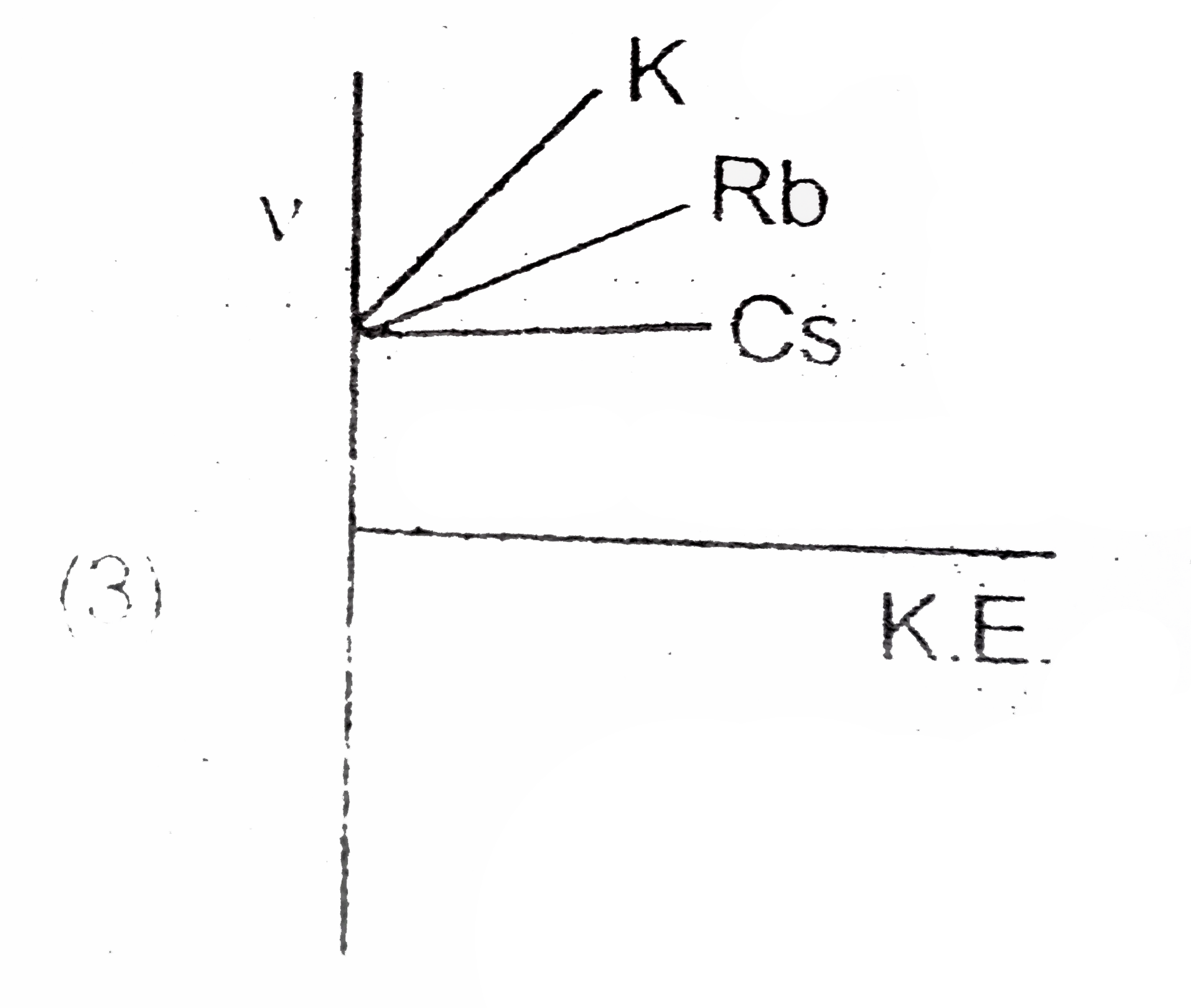A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -I) Subjective Type Questions|7 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -D) Assertion-Reason Type Questions|15 VideosSURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - C (Assertion - Reason type questions)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-STRUCTURE OF ATOM -ASSIGNMENT ( SECTION -J) Aakash Challengers Questions
- How many maximum spectral lines are possible if electron is present in...
Text Solution
|
- The correct graph regarding v vs KE. ( Incident)
Text Solution
|
- Maximum electron in 1^(st) shell having m(l) =2 will be
Text Solution
|
- What about degeneracy of 2p orbitals in a magnetic field?
Text Solution
|
- In electric field which have maximum angle of deflection? ( Assume all...
Text Solution
|
- Choose the pair in which after filling of electron , energy sequence i...
Text Solution
|
- The orbital having zero probability of finding electron on the surface...
Text Solution
|
- Given graph may belong with
Text Solution
|
- Considering the graph of 1s , choose the correct option:
Text Solution
|
- An electron in hydrogen jumps to a shell in which four fold degeneracy...
Text Solution
|
- Largest wavelength is associated with which one of the following . If ...
Text Solution
|
- Which of the following have highest ionisation energy ?
Text Solution
|