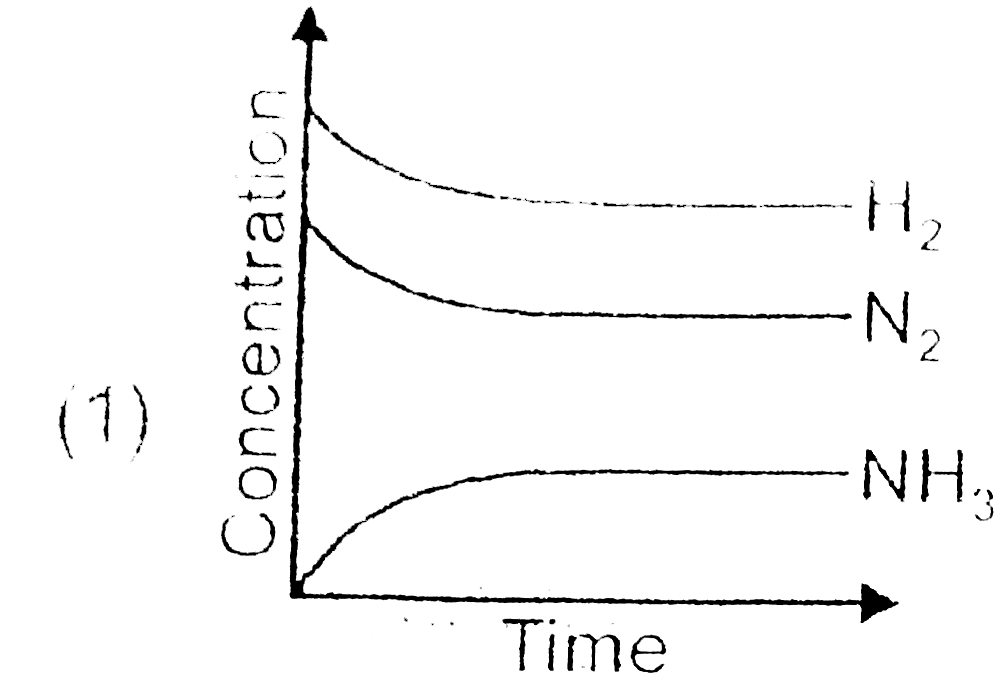
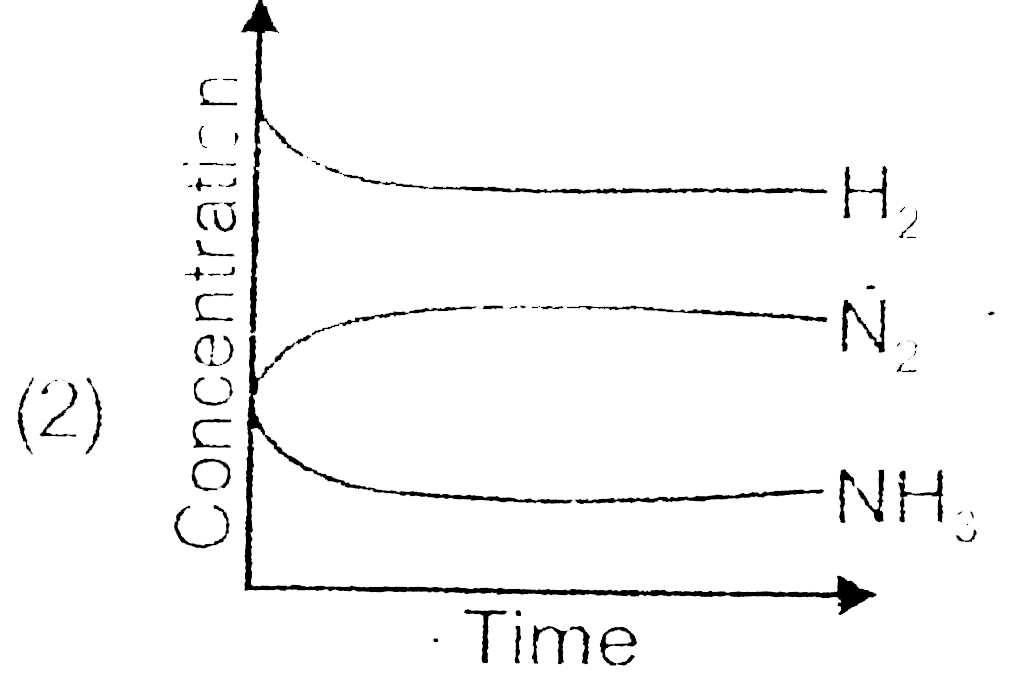
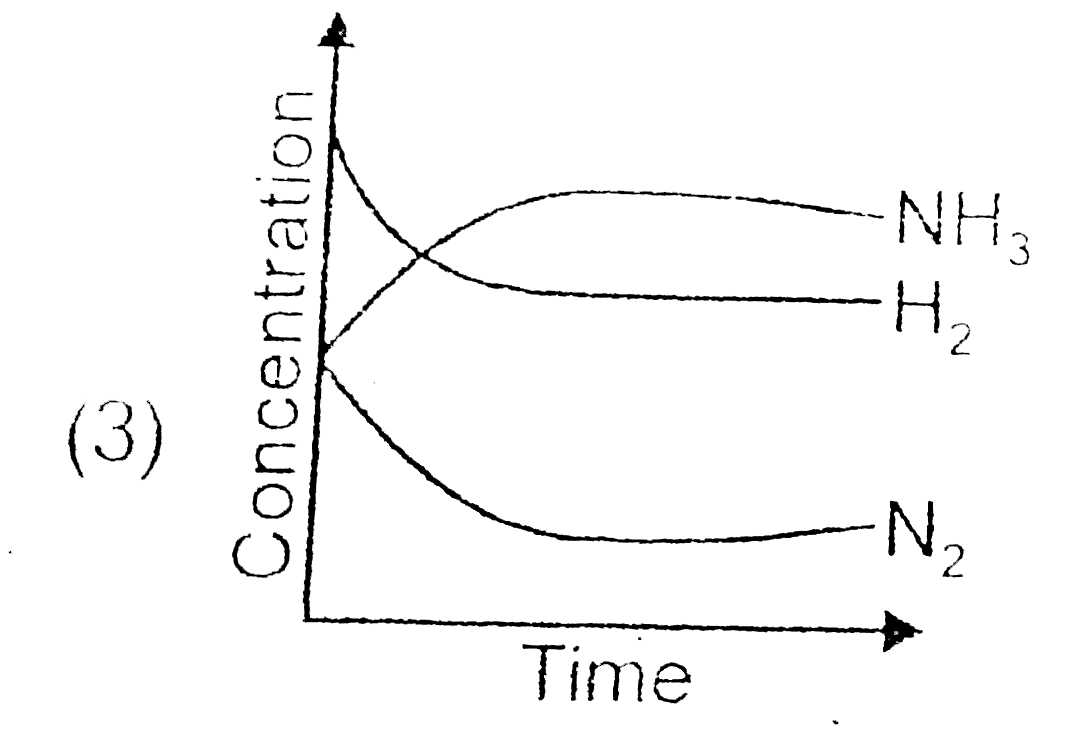
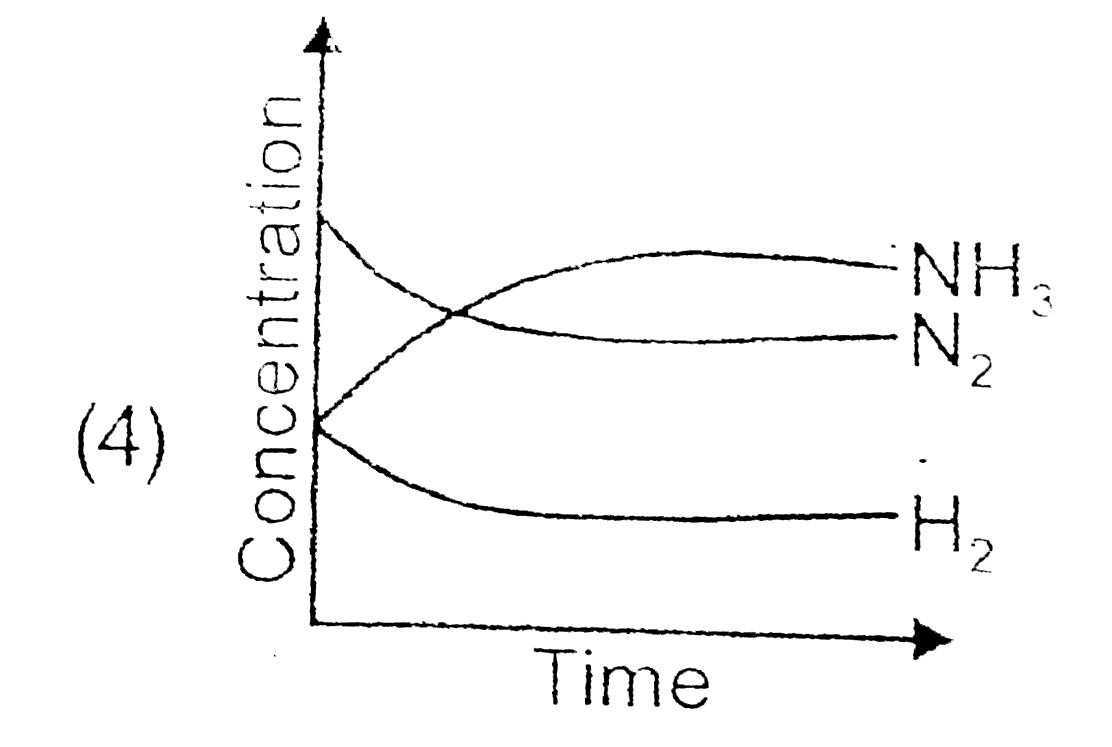
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION-B)(OBJECTIVE TYPE QUESTIONS (ONE OPTION IS CORRECT)|20 VideosEQUILIBRIUM
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION-C) (OBJECTIVE TYPE QUESTIONS (MORE THAN ONE OPTION ARE CORRECT)|10 VideosEQUILIBRIUM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -D)|20 VideosENVIRONMENTAL CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D) (Assertion - Reason Type Questions)|4 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-D) Assertion-Reason Type Question|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-EQUILIBRIUM-Assignment (SECTION-A) (SUBJECTIVE TYPE QUESTIONS(ONE OPTION IS CORRECT)
- If K(1) and K(2) are respective equilibrium constants for two reactio...
Text Solution
|
- For the reaction CO(g)+(1)/(2) O(2)(g) hArr CO(2)(g),K(p)//K(c) is
Text Solution
|
- 500 ml vessel contains 1.5 M each of A, B, C and D at equlibrium. If 0...
Text Solution
|
- 9.2 grams of N(2)O(4(g)) is taken in a closed one litre vessel and hea...
Text Solution
|
- For the synthesis of ammonia by the reaction N(2)+3H(2)hArr2NH(3) in t...
Text Solution
|
- The equilibrium: P(4)(g)+6Cl(2)(g) hArr 4PCl(3)(g) is attained by ...
Text Solution
|
- For the hypothetical reactions, the equilibrium constant (K) value are...
Text Solution
|
- Partial pressure of O(2) in the reaction 2Ag(2)O(s) hArr 4Ag(s)+O(2)...
Text Solution
|
- For the following gases equilibrium, N(2)O(4)(g)hArr2NO(2)(g) K(p) i...
Text Solution
|
- NH(4)COONH(2)(s)hArr2NH(3)(g)+CO(2)(g) If equilibrium pressure is 3 at...
Text Solution
|
- The following two reactions: i. PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) ...
Text Solution
|
- When hydrogen molecules decompose into its atoms, which conditions giv...
Text Solution
|
- Equilivalent amounts of H(2) and I(2) are heated in a closed vessel ti...
Text Solution
|
- The dissociation constants for acetic acid and HCN at 25^(@)C are 1.5x...
Text Solution
|
- Hg(2)Cl(2)(g) in saturated aqeous solution has equilibrium constant eq...
Text Solution
|
- K(rho) for the following reaction will be equal to 3Fe(s)+4H(2)O(g)hAr...
Text Solution
|
- Which of the following factors will favour the reverse reaction in a c...
Text Solution
|
- The pH of 10^(-8)M solution of HCl in water is
Text Solution
|
- The pH of 0.05 M solution of a strong dibasic acid is
Text Solution
|
- Among the following the one which does not represent a conjugate acid-...
Text Solution
|