Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Exercise|20 VideosSURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - A (Objective type questions)|39 VideosSURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION - I|5 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -J) Aakash Challengers Questions|12 VideosTEST 1
AAKASH INSTITUTE ENGLISH|Exercise EXAMPLE|134 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-SURFACE CHEMISTRY -ASSIGNMENT SECTION - J
- At 1 atm and 273 K the volume of nitrogen gas required to cover a samp...
Text Solution
|
- 5 mL of 0.3 M acetic acid is shaken with 5 g of activated charcoal. Th...
Text Solution
|
- Around 20% surface sites have adsorbed N(2). On heating N(2) gas evolv...
Text Solution
|
- Calculate the surface area of a catalyst that adsorbs 10^3 cm^3 of N2 ...
Text Solution
|
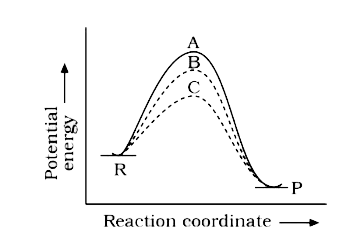
- If a homogeneous catalytic reaction follows three alternative paths A,...
Text Solution
|
- Suppose we have a cube of 1.00 cm length. It is cut in all three direc...
Text Solution
|