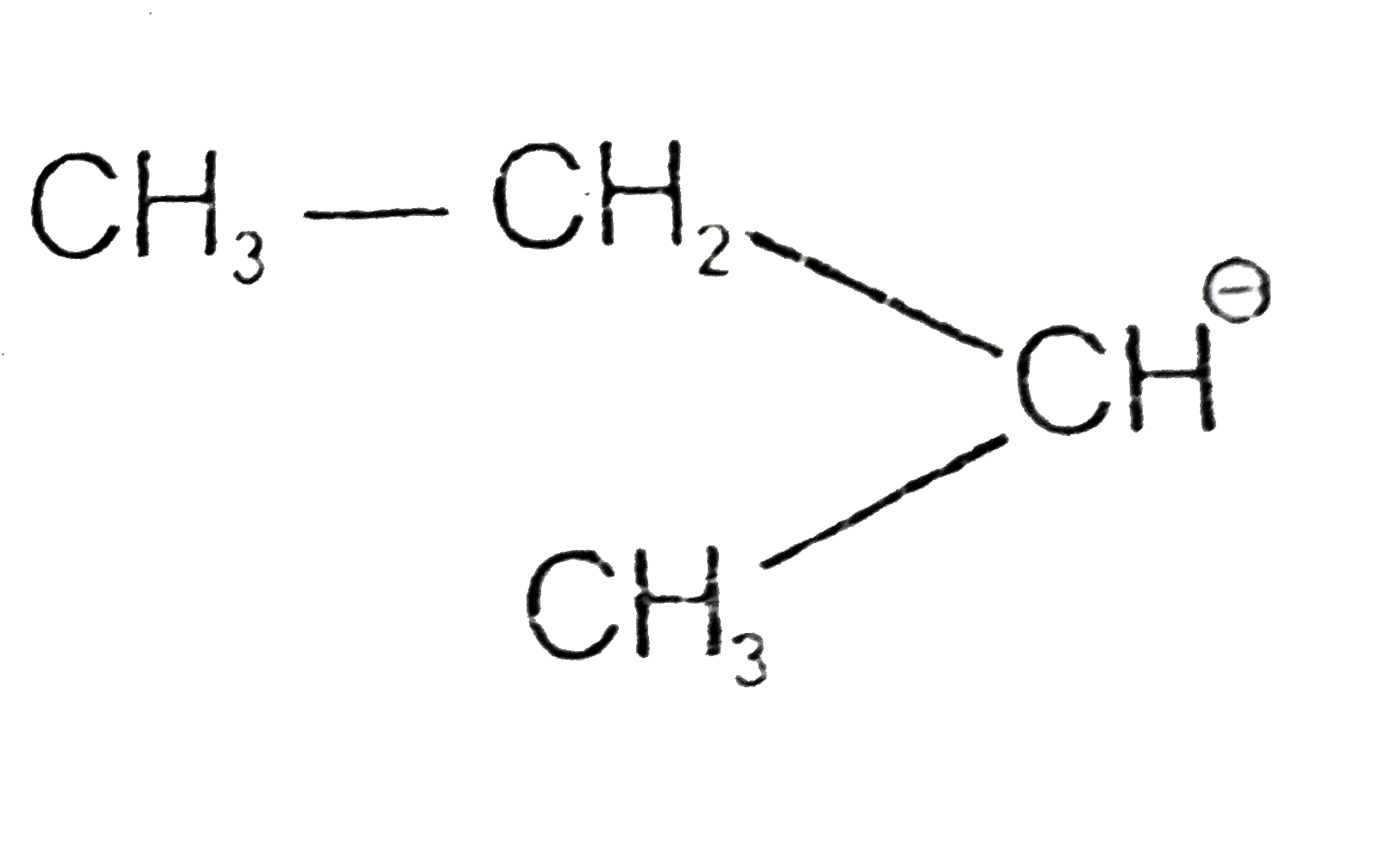
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-B OBJECTIVE TYPE QUESTION (ONE OPTION IS CORRECT)|37 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-C OBJECTIVE TYPE QUESTION (MORE THAN ONE OPTIONS ARE CORRECT)|18 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise ILLUSTRATION|3 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|15 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-ASSIGNMENT SECTION-A COMPETITION LEVEL QUESTIONS
- The IUPAC name of CH(3)-overset(O)overset(||)(C)-CH(2)-overset(OH)ov...
Text Solution
|
- The IUPAC name of tertiary butyl chloride is
Text Solution
|
- Most stable carbanion among the following is
Text Solution
|
- In which of the following, homolytic bond fission takes place :
Text Solution
|
Text Solution
|
- Aldehyde and ketones are
Text Solution
|
- Diethyl ether and methyl n propyl ether are
Text Solution
|
- +I effect is shown by:
Text Solution
|
- Electromeric effect:
Text Solution
|
- The reaction intermediate produce by homolytic cleavage of bond is cal...
Text Solution
|
- Ammonia is iso-structural with:
Text Solution
|
- A mixture of camphor and benzoic acid can be separated by
Text Solution
|
- In Kjeldahl's method, nitrogen present is estimated as
Text Solution
|
- In sodium fusion test of organic compounds, the nitrogen of an organic...
Text Solution
|
- In Dumas' method, the gas (or vapor) which is collected in the nitrome...
Text Solution
|
- 0.2 g of an organic compound on comptete combustion produces 0.18 g of...
Text Solution
|
- In a Lassaigne's test for sulphur in the organic compound with sodium ...
Text Solution
|
- Lassaigne's test for the detection of nitrogen will fail in case of :
Text Solution
|
- The Lassaigne's extract is boiled with dil. HNO(3) before testing for ...
Text Solution
|
- The purity of an organic compound is determined by
Text Solution
|