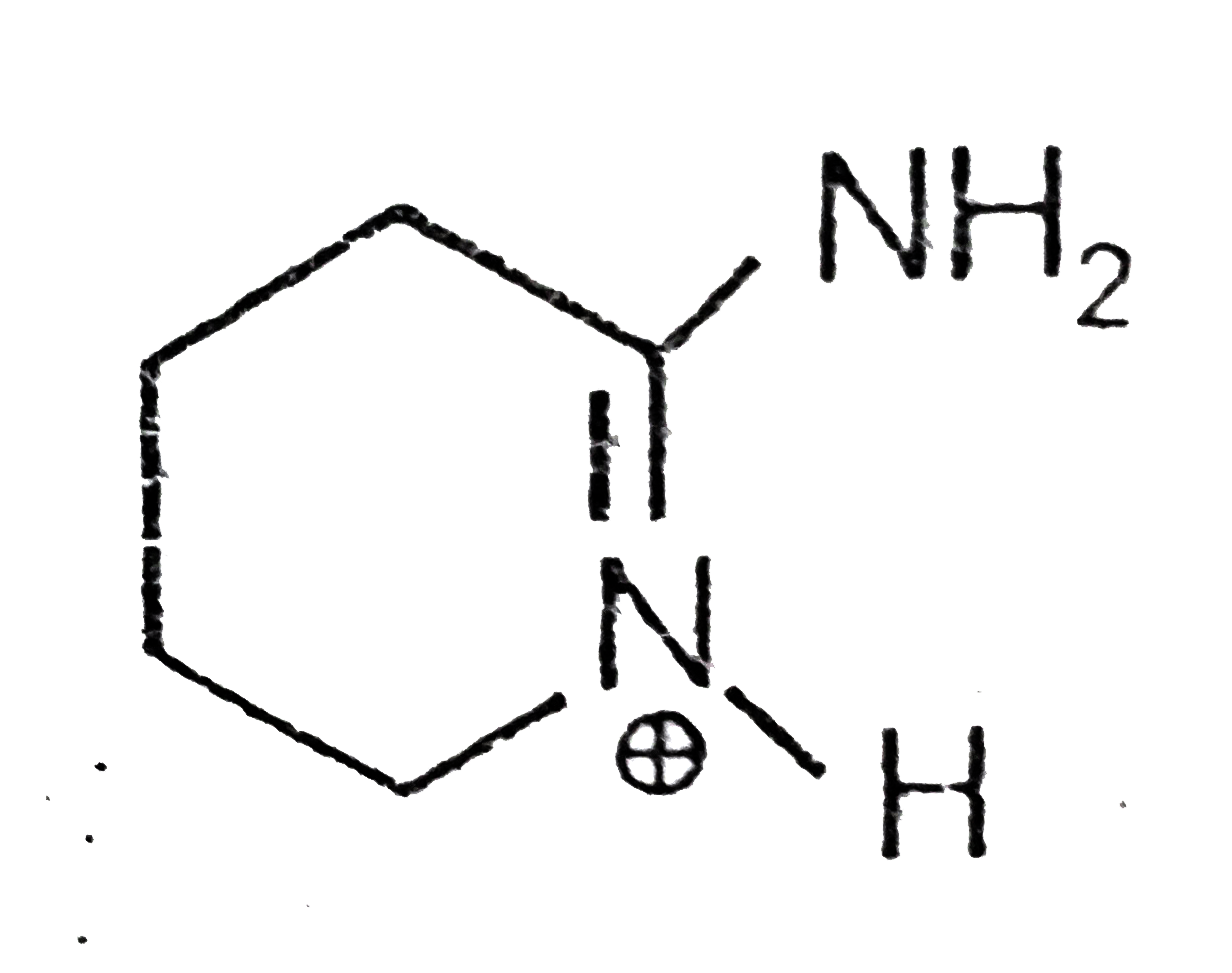
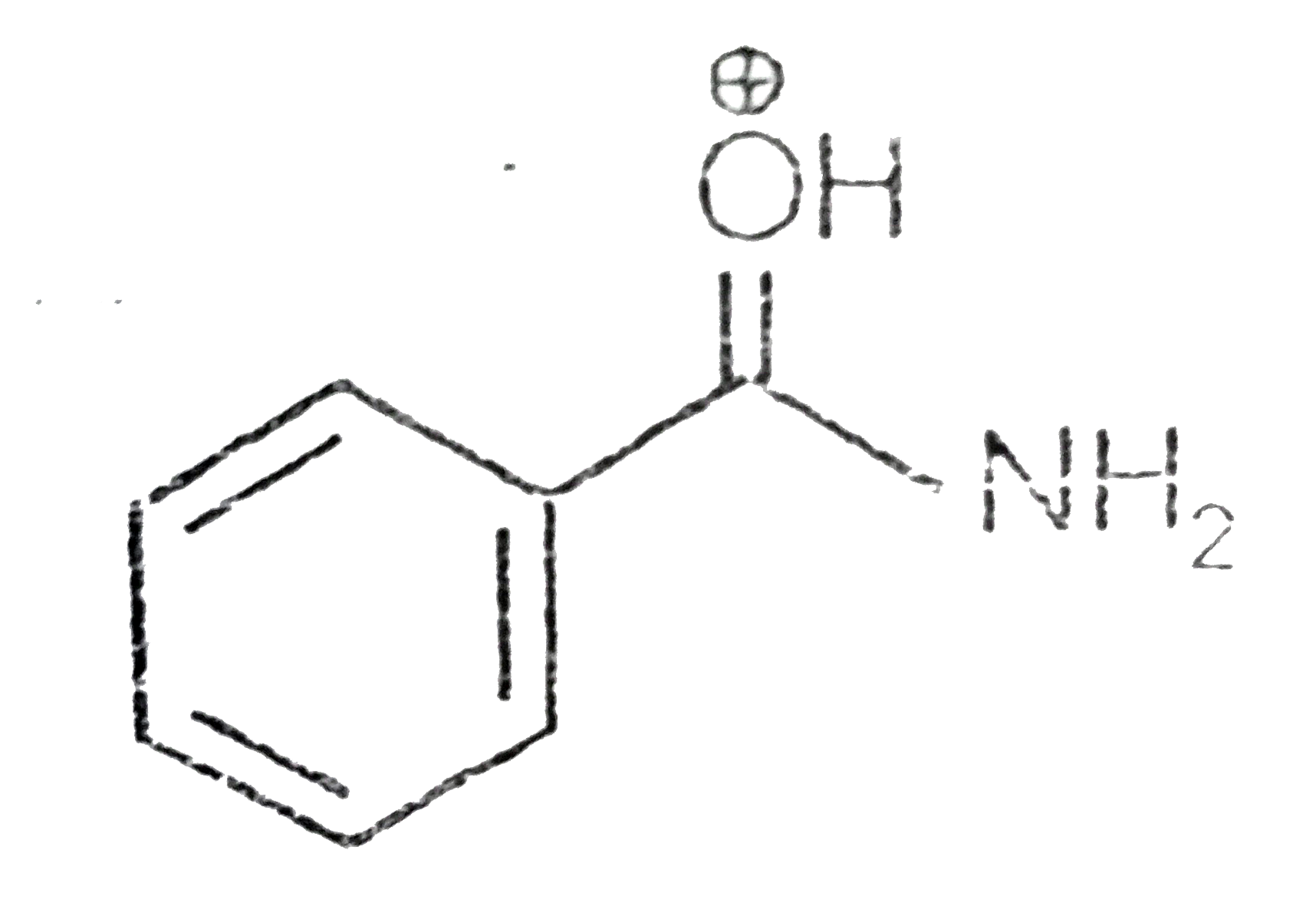
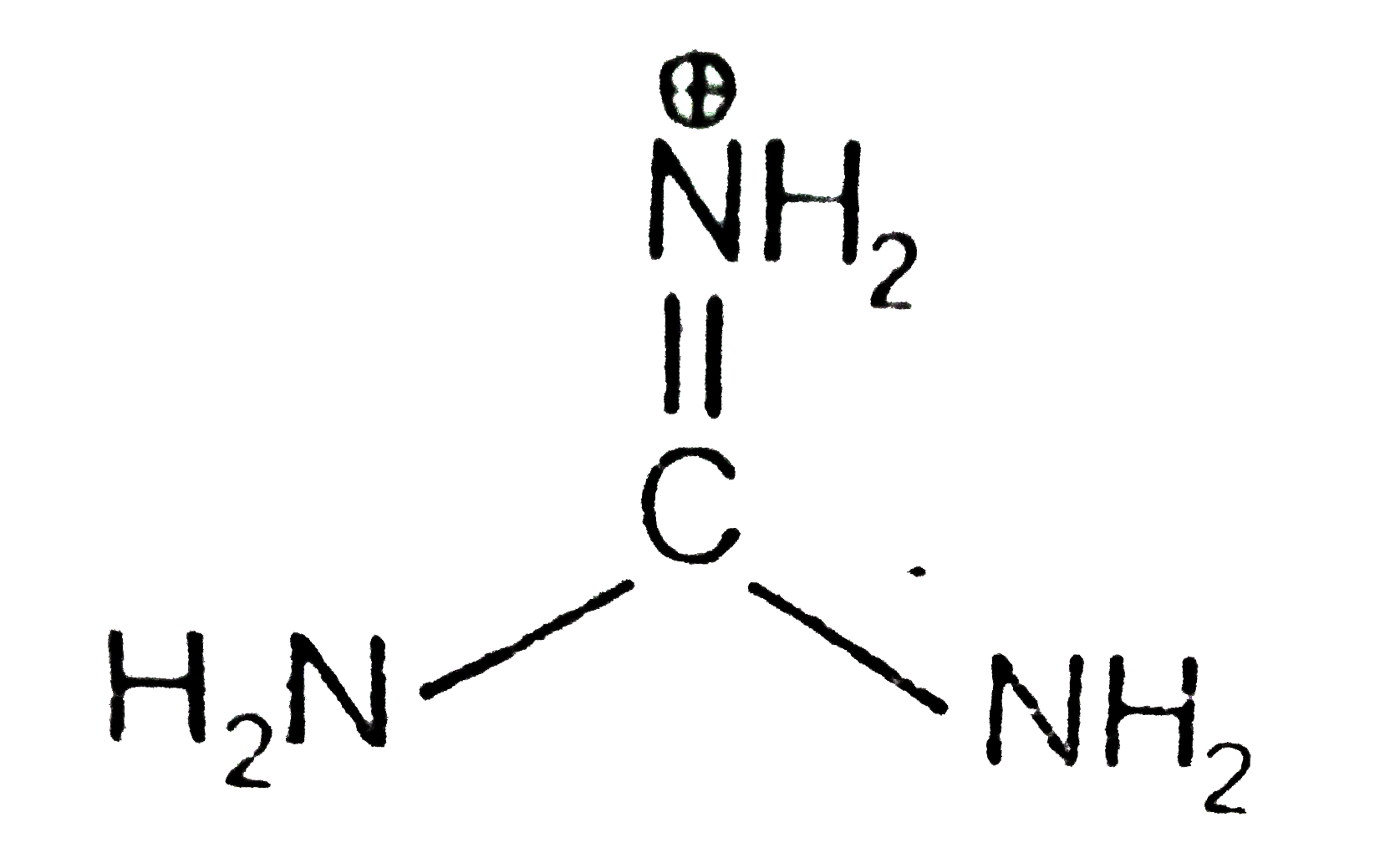
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-C OBJECTIVE TYPE QUESTION (MORE THAN ONE OPTIONS ARE CORRECT)|18 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-D LINKED COMPREHENSION TYPE QUESTIONS|7 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION-A COMPETITION LEVEL QUESTIONS|50 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|15 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-SECTION-B OBJECTIVE TYPE QUESTION (ONE OPTION IS CORRECT)
- Which will have highest melting point?
Text Solution
|
- Which of the following is the strongest base in water?
Text Solution
|
- Which of the following is most acidic?
Text Solution
|
- Isomers which can be interconverted through rotation around a single b...
Text Solution
|
- In the given anion,-ve charge is delocalized on
Text Solution
|
- The compounds are
Text Solution
|
- Least stable carbocation among the following is
Text Solution
|
- Which of the following compound will give blood red colour while doing...
Text Solution
|
- The most stable free radical is
Text Solution
|
- Which of the following group will have strongest electron donating mes...
Text Solution
|
- Among the following the most stable carbocation
Text Solution
|
- Most acidic species among the following is
Text Solution
|
- Which of the following double bond in the given molecule is most react...
Text Solution
|
- Which of the following hydrocarbon is most stable ?
Text Solution
|
- Resonance is not possible in
Text Solution
|
- Which of the following compounds will have highest enolic content?
Text Solution
|
- Among the following The correct order of reactivity of chloride is
Text Solution
|
- Which of the following represents the correct order of stability of th...
Text Solution
|
- Which of the following organic molecule can not form hydrogen bond in ...
Text Solution
|
- Correct stability order of the given free radicals is
Text Solution
|