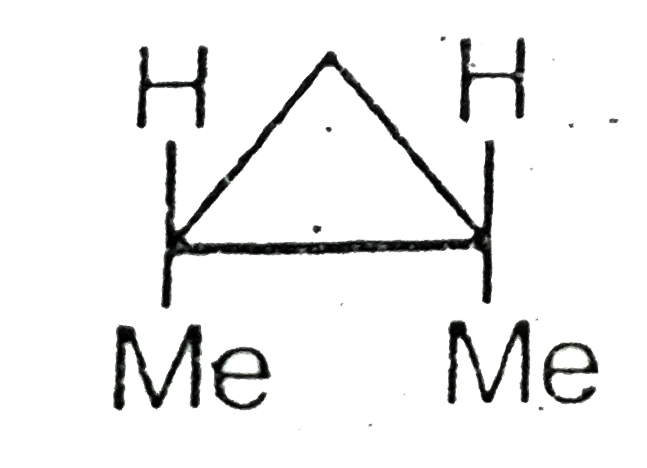
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-D LINKED COMPREHENSION TYPE QUESTIONS|7 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-E ASSERTION-REASON TYPE QUESTIONS|14 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-B OBJECTIVE TYPE QUESTION (ONE OPTION IS CORRECT)|37 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|15 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-SECTION-C OBJECTIVE TYPE QUESTION (MORE THAN ONE OPTIONS ARE CORRECT)
- Which of the following bicyclic compounds are isomers?
Text Solution
|
- Species which will exhibit geometrical isomerism among the following i...
Text Solution
|
- Which of the following molecular formula will exhibit functional isome...
Text Solution
|
- Out of the given isomeric hydrocarbons which will undergoes rearrangem...
Text Solution
|
- Which of the following is stablised by overlapping of p-orbitals?
Text Solution
|
- Formic acid is more acidic than
Text Solution
|
- Which of the following will have C=O bond length almost similar to C-O...
Text Solution
|
- Dichloro ethene shows
Text Solution
|
- A compound having molecular formula C(4)H(10)O can show
Text Solution
|
- Consider the following compounds Which of the following statements are...
Text Solution
|
- Which of the following correctly represent the acidic strenth of given...
Text Solution
|
- Which of the following correctly represents the stability of reactive ...
Text Solution
|
- Keto-enol Tautomerism is observed in
Text Solution
|
- Which of the following can exhibit geometrical isomerism?
Text Solution
|
- The compounds which cannot react with NaOH is/are
Text Solution
|
- The hybridisation of N is correctly given in
Text Solution
|
- Which of the following Lewis structures are valid resonating structure...
Text Solution
|
- In the given compound, hybridization of the Carbon atom with positive ...
Text Solution
|