Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-J AAKASH CHALLENGERS QUESTIONS|10 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|15 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-TRY YOURSELF
- Write the IUPAC names of the following compounds (i) CH(3)-CH(2)-ove...
Text Solution
|
- Derive the structure of (i) 2-Chlorohexane, (ii) Pent-4-en-2-ol, (iii)...
Text Solution
|
- Draw all the possible isomers of C(4)H(8)O (containing carbonyl group)
Text Solution
|
- What is the relation between 2-methyl propanaol-1 and 2-methoxy propan...
Text Solution
|
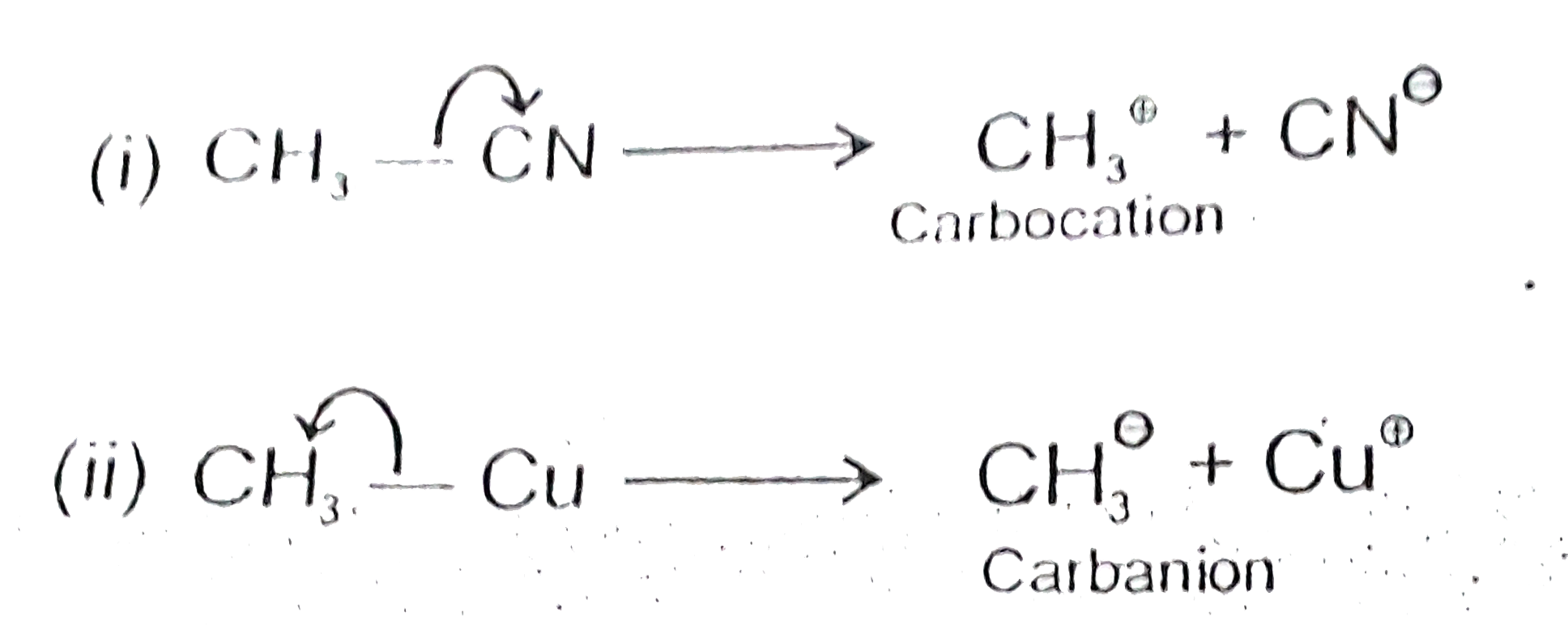
- Using curved-arrow notation, show the formation of reactive intermedi...
Text Solution
|
- Classify the following molecules/ions as nucleophiles or electrophiles...
Text Solution
|
- Draw the resonating strcuture for C(6)H(5)CHO compound.
Text Solution
|
- Arrange the following carbocations in increasing order of stability an...
Text Solution
|
- On complete combustion, 0.246 g of an organic compound gave 0.198g of ...
Text Solution
|
- 0.2613g of an organic compound on combustion in oxygen gave 0.8844 g o...
Text Solution
|
- 0.2313 g of an organic substance gave 40 ml of moist nitrogen measured...
Text Solution
|
- 0.27g of an organic compound gave on combustion 0.396 of CO(2) 0.216g ...
Text Solution
|
- 0.2g of an organic compound of kjedahl's analysis gave enough ammonia ...
Text Solution
|
- A sample of 0.50 g of an organic compound was treated according to Kje...
Text Solution
|
- In Carius method of estimation of halogen, 0.15 g of an organic compou...
Text Solution
|
- 0.525g of an organic compound gave 0.356g of silver chloride by a halo...
Text Solution
|
- 0.2595 g of an organic substance, when treated by carius method gave ...
Text Solution
|
- In sulphur estimation, 0.157 g of an organic compound gave 0.4813 g of...
Text Solution
|
- 0.092 g of an organic compound containing phosphorus gave 0.111 g Mg(2...
Text Solution
|
- 0.12 gm of an organic compound containing phosphorus gave 0.22 gm of M...
Text Solution
|